The micro-alloying effects of Y on the microstructure, mechanical properties, and bio-corrosion behavior of Mg69- xZn27Ca4Y x ( x = 0, 1, 2 at.%) alloys were investigated through X-ray diffraction, compressive tests, electrochemical treatments, and immersion tests. The Mg69Zn27Ca4 alloy was found to be absolutely amorphous, and its glass-forming ability decreased with the addition of Y. The Mg68Zn27Ca4Y1 alloy exhibited an ultrahigh compressive strength above 1010 MPa as well as high capacity for plastic strain above 3.1%. Electrochemical and immersion tests revealed that these Y-doped Mg-Zn-Ca alloys had good bio-corrosion resistance in simulated body fluid (SBF) at 37 °C. The results of the cytotoxicity test showed high cell viabilities for these alloys, which means good bio-compatibility.
In recent years, metallic glasses have drawn a great deal of attention and are promising materials for structural and functional applications. Many successful amorphous systems[1], [2], [3], [4] and [5], including Zr, Fe, Mg, Ni, Cu, and Pd based alloys, have been developed over the past few decades. Among these amorphous alloys, Mg-based bulk metallic glasses (BMGs) have great potential to be used as implant materials, for their high specific strength, low cost and good biocompatibility and biodegradation properties[6], [7] and [8].
However, magnesium alloys as implant materials are facing two challenges[9] and [10] (1): Higher intrinsic strength is required as their strength is reduced during the degradation process (2). The high dissolution rates of Mg and Mg alloys are mainly due to the presence of impurities, second phases or intermetallics, which promotes galvanic corrosion and results in rapid disintegration. Therefore, Mg-based BMGs with enhanced mechanical and corrosion properties are increasingly being paid attention because of their structural and chemical homogeneity and the absence of second crystal phases[9] and [11].
Of all the Mg-based amorphous alloys, Mg-Zn alloys are expected to be better used in biomedical application than Mg-Cu, Mg-Ni and other Mg-based alloys. It is reported that the recommended intake of magnesium (Mg) for an adult is about 400 mg/day. The excess magnesium cations are harmlessly excreted in the urine and the presence of Mg in the bone is beneficial to bone strength and growth[12]. Zinc is an abundant nutritionally essential element in the human body and plays a physiological role in the regulation of bone metabolism[13]. Moreover, zinc has a stimulatory effect on bone formation, mineralization and an inhibitory effect on bone resorption. Besides Mg and Zn elements, calcium is also a major component of the human bone. The recommended calcium intake for an adult is 1000 mg/day[14]. Magnesium alloys have low density (1.74-2 g/cm3) and Young's modulus (41-45 GPa), which are almost similar to the natural bone (1.8-2.0 g/cm3, 5-23 GPa)[15]. Some researches[14] and [16] show that both Mg-Zn and Mg-Ca binary alloys have acceptable biocompatibility and can be considered new biodegradable implant materials.
More recently, numerous efforts have been made to study the Mg-Zn-Ca metallic glass system and its potential for biomedical application[9], [17], [18] and [19]. Zberg et al.[17] found that there was an obvious reduction in hydrogen evolution in Zn-rich Mg-Zn-Ca glasses and the glasses exhibited a similar level of biocompatibility as their crystalline counterparts. The Mg68Zn28Ca4 glassy alloy was also reported to exhibit a compressive strength of 828 MPa by Li et al.[11], which is much higher than that of the crystalline Mg-4.0Zn-0.5Ca alloy (273 MPa)[20]. Unfortunately, all these Mg-Zn-Ca BMGs always fracture in the elastic regime without observable plastic deformation due to their inhomogeneous deformation behavior[21], which limits their application. To resolve this limitation, a ductile, phase-reinforced, glassy alloy matrix formed in-situ has recently been used [2], [3], [4], [21] and [22].
Y is known to improve the manufacturability of metallic glass alloys by scavenging for oxygen impurities via the formation of innocuous yttrium oxides[5]. Y could also refine the microstructure of magnesium alloys as well as reduce the corrosion rate, indicating that Y can improve the mechanical properties and corrosion resistance of magnesium alloys[23] and [24]. The cytotoxicity and hemolysis tests showed that the Mg-Y alloy caused no significant toxicity to osteoblasts and induced less than 5% hemolysis, indicating that the effective alloying element Y promotes the biomedical application of magnesium alloy[25].
In the current study, Y was added to an Mg-Zn-Ca glassy alloy. X-ray diffraction (XRD), compressive tests, electrochemical treatments, immersion tests and cytotoxicity test were used to reveal the effects of Y on the microstructure, mechanical, bio-corrosion and biocompatibility properties of Mg-Zn-Ca bulk metallic glass.
The ingots of Mg69-xZn27Ca4Yx (x = 0, 1 and 2 at.%) were obtained by melting the mixture of pure Mg (99.9 wt%), Zn (99.9 wt%), Mg-Ca master alloy (30 wt% Ca), and Mg-Y master alloy (25.7 wt% Y) in an induction furnace with argon in the atmosphere. Then the Mg69-xZn27Ca4Yx master alloys were re-melted and injected into a copper-mold with a diameter of 1.5 mm. The samples for immersion and electrochemical tests were wet ground through successive grades of silicon carbide emery papers of up to 1400 grit, washed with distilled water, degreased with acetone and then dried in air.
The microstructure of the alloys was studied through XRD (Rigaku D/MAX-2500PC) with CuKα radiation and scanning electron microscopy (SEM, TESCAN VEGA Ι Ι LMU). The XRD pattern was analyzed with MDI Jade5.0 software. The thermal stability of the alloys was investigated by differential scanning calorimetry (DSC, PE-7) at the heating rate of 20 K/min. The compressive specimens with an aspect ratio of 2:1 were cut using a low-speed diamond saw and were then polished to guarantee parallelism and perpendicularity prior to the uniaxial compressive test. The compressive tests were carried with a SANS CMT5105 testing machine at the strain rate of 10-4 s-1.
The corrosion behavior analysis was conducted with an electrochemical workstation (CHI660C, China) at 37 ° C in simulated body fluid (SBF[9], NaCl 8.035 g/L, NaHCO3 0.355 g/L, KCl 0.225 g/L, K2HPO4· 3H2O 0.231 g/L, MgCl2· 6H2O 0.311 g/L, CaCl2 0.292 g/L, Na2SO4 0.072 g/L, (HOCH2)3CNH2 6.118 g/L). Electrochemical measurements were performed through a typical three-electrode cell with a platinum plate as counter electrode, a saturated calomel electrode as reference electrode and the sample with an exposed surface as working electrode. All samples were cut from the copper-mold injected samples and the as-cast pure Mg. These samples were molded into an epoxy resin with only one side of Φ 1.5 mm exposed for the test. Prior to the potentiodynamic polarization test, the samples were kept in the solution for 4000 s in order to stabilize the open circuit potential (OCP). The measurements swept from the initial potential of -2.0 V/SCE at a constant scan rate of 1 mVs-1 to the final potential of approximate -0.9 V/SCE. The corrosion potential (Ecorr) and corrosion current density (icorr) were fitted using the CorrView software in the mode of Tafel (traditional).
Cylindrical samples with a dimension of Φ 1.5 mm × 10 mm were cut from the copper-mold injected samples and the as-cast pure Mg to prepare for the immersion test. All samples were immersed in SBF (45 h for hydrogen evolution and 85 h for pH measurement) through a water bath under the constant temperature of 37 ° C. The initial pH value of the SBF solution was 7.3. The volume of H2 was measured over time. After the immersion test, the samples were gently rinsed with distilled water and dried in air. A Vega II LMU SEM was utilized to observe the surface morphologies. All the tests were performed in duplicate to guarantee the reproducible results.
Osteoblasts harvested from neonatal rat calvaria with a sequential collagenase digestion method were cultured in Dulbecco's modified Eagle's medium (DMEM), 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 mg/mL streptomycin at 37 ° C in a humidified atmosphere of 5% CO2. Osteoblasts at the 3rd passage were used in the following experiments. The cytotoxicity test was carried out by the indirect contact.
The extracts of the samples were prepared by the DMEM serum free medium, with the surface area/extraction medium ratio 1 cm2/mL in a humidified atmosphere with 5% CO2 at 37 ° C. After 72 h incubation, the supernatant fluid was withdrawn and centrifuged to prepare the extraction medium. Then the extraction medium was serially diluted to 10% concentration. The extraction medium was refrigerated at 4 ° C before the cytotoxicity test. The control groups involved the use of DMEM medium as negative control in this experiment. Cells were incubated in 96-well cell culture plates at an initial density of 5 × 103 cells/well with 100 μ L DMEM medium and incubated for 24 h to allow attachment. Then the medium was replaced with 100 μ L of extracts. After incubating the cells in a humidified atmosphere with 5% CO2 at 37 ° C for 3 days, 10 μ L cell counting kit (CCK-8) was added and then the samples were incubated with CCK-8 in DMEM in at 37 ° C for 4 h. The spectrophotometrical absorbance (OD) of the samples was measured by microplate reader (Bio-RAD 680) at 570 nm. The cell viability was expressed as relative growth rate (RGR) calculated according to the following formula:
RGR=ODsample/ODcontrol× 100%RGR=ODsample/ODcontrol× 100% 
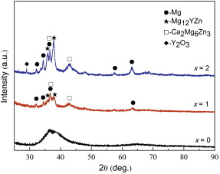
Fig. 1 shows the XRD patterns of the as-injected Mg69-xZn27Ca4Yx (x = 0, 1 and 2 at.%) alloy rods with the diameter of 1.5 mm. The Mg69Zn27Ca4 alloy exhibits typical amorphous features as indicated by the absence of detectable crystalline diffraction peaks. However, the Mg68Zn27Ca4Y1 alloy exhibits sharp peaks superimposed on a broad diffraction peak, implying the co-existence of crystalline and amorphous phases. The crystalline phases are further confirmed to be composed of Mg solid solution, Ca2Mg6Zn3, and Mg12YZn. With the addition of 2 at.% Y, the intensity of these crystalline phases increases further, and the broad peaks disappear gradually. Because of the high content of Y, Y2O3 was detected in Mg67Zn27Ca4Y2. From the XRD results, it is suggested that the micro-alloying Y could ruin glass-forming ability (GFA) because of the limited GFA of the Mg-Zn-Ca metallic glass [11].
Fig. 2 displays the DSC curves of the three alloys and reveals the glass transition and crystallization behavior. It could be observed that the Mg69Zn27Ca4 alloy visibly presents glass transition and multistep crystallization. The glass-forming ability of Mg69Zn27Ca4 alloy is improved greatly compared with Mg68Zn28Ca4 alloy which has no glass transition characteristics in our previous study[26]. However, the thermal behavior changes remarkably even with the scantling of Y as indicated by the disappearance of glass transition temperature Tg, the dramatic rise of the crystallization temperature Tx, and the rapid decrease of the crystallization heat Δ H for the Mg68Zn27Ca4Y1 alloy. The main thermal parameters of these three alloys are summarized in Table 1.
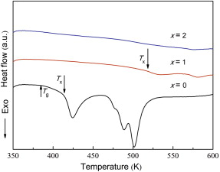 | Fig. 2 DSC curves of the crystallization of Mg69-xZn27Ca4Yx (x = 0, 1, and 2 at.%) alloys at a heating rate of 20 K min-1. |
| Table 1 Critical parameters of thermal, mechanical properties and corrosion resistance of Mg69-xZn27Ca4Yx (x = 0, 1, 2 at.%) alloys, ZK60 and pure Mg. Note that Δ Tm represents the difference between the melting temperature Tm and the liquid temperature Tl, σ b represents the ultimate compress stress and ε represents the elongation |
Fig. 3 shows the SEM backscattered electron images of the Mg69-xZn27Ca4Yx (x = 0, 1 and 2 at.%) alloys. There is no distinct contrast in the backscattered electron scanning images, which means that the Mg69Zn27Ca4 alloy has a pure amorphous structure again ( Fig. 3(a)). However, the SEM images of the Y-doped Mg-Zn-Ca samples consist of several different regions ( Fig. 3(b) and (c)). The Mg68Zn27Ca4Y1 alloy consists of small dark areas surrounded by gray regions and scattered white dendrites, which are shaped like snowflakes. All these phases are fine and uniformly distributed on the Mg matrix. The microstructure and phase distribution exhibited by the Mg67Zn27Ca4Y2 alloy appear to be similar to Mg68Zn27Ca4Y1 alloy. However, the white snowflake phases are much larger in the Mg67Zn27Ca4Y2 alloy compared with the Mg68Zn27Ca4Y1 alloy.
To identify the composition of the crystalline phase in the different regions observed in the SEM images, energy dispersive spectrometry (EDS) spectrums were obtained from the Y-doped alloy. Table 2 summarizes the EDS results of the different regions of the Mg68Zn27Ca4Y1 alloy. The results show that the gray regions are rich in Mg, Zn, Ca and the atomic percentages are similar to that of the matrix of Mg69Zn27Ca4 (56.51 at.% Mg, 3.36 at.% Ca, 40.13 at.% Zn), which means that the gray regions are the amorphous matrix. The white snowflake phases are composed of high Mg, high Ca but low Zn content. Also, much of Y could be found in this area. Based on the atomic weight, it can be assumed that the white snowflake phases correspond to the mixture of the Ca2Mg6Zn3 phase and Mg12YZn phase. The atomic percentages of Mg, Zn, Ca in dark areas are similar to that of the matrix. The difference is that a little of Y exists. It is suggested that this dark region is a transition zone between the matrix and the dendrites phase.
| Table 2 EDS results for Mg68Zn27Ca4Y1 alloys |
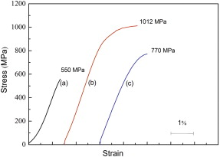
Fig. 4 shows the compressive stress-strain curves which shift apart from each other to avoid overlapping of the alloys. The results show that the Mg69Zn27Ca4 glassy alloy has greater strength than the traditional crystalline Mg-4.0Zn-0.5Ca alloy (273 MPa)[20]. The Mg69Zn27Ca4 glassy alloy also exhibits linear elastic behavior and it only fails in the absence of macroscopic yielding and plastic strain. However, Y can significantly improve fracture strength. Fracture strength increases from 550 to 1012 MPa (80% increment) with the addition of 1 at.% Y, which is 4 times higher than that of traditional magnesium alloys and 26% higher than Mg-Zn-Ca metallic glass as reported by Li et al.[11]. The Mg68Zn27Ca4Y1 alloy also exhibits excellent capacity for plastic strain, above 3.1%, which is 2.5 times higher than the capacity of Mg-Zn-Ca metallic glass[11]. In a word, the Mg68Zn27Ca4Y1 alloy shows the best comprehensive mechanical properties in this experiment. The mechanical properties of these alloys are also listed in Table 1.
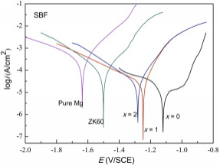
A comparison of the polarization curves of the Y-doped Mg69Zn27Ca4 alloy in SBF solution at 37 ° C is shown in Fig. 5. Generally, cathodic polarization curves can be assumed to represent the cathodic hydrogen evolution through water reduction, whereas the anodic polarization curves represent the dissolution of magnesium[18]. The Mg69Zn27Ca4 glassy alloy is spontaneously passivated at a passivation interval and a very low passivation current density, allowing the formation of a good passive film. For comparison, the ZK60 and pure Mg were also tested in the same condition. Although the passivation interval disappears, the Mg68Zn27Ca4Y1 and Mg67Zn27Ca4Y2 alloys still have much more noble corrosion potential (Ecorr) and lower corrosion current density (icorr) than the pure Mg and ZK60 alloys. The main parameters of the bio-corrosion resistance of these alloys are also summarized in Table 1. The corrosion current density (icorr) of the Mg69Zn27Ca4 glassy alloy is 10-5.56 A/cm2, which exhibits only 1/40 of pure Mg. Similar results were reported that an increase in the cooling rate could improve the corrosion resistance of the Mg97.25Zn0.75Y2 alloy [27].
The variation of hydrogen evolution volume as a function of immersion time is shown in Fig. 6. All the samples exhibit an increase in the volume of H2 in the span of 45 h, which means an increment in hydrogen evolution rate[24]. The hydrogen evolution volume of the Mg69-xZn27Ca4Yx alloys and pure Mg could be ranked in an increasing series as follows: Mg69Zn27Ca4 < Mg68Zn27Ca4Y1 < Mg67Zn27Ca4Y2 < pure Mg. It can be concluded that the Mg69-xZn27Ca4Yx alloys have a much lower corrosion rate than pure Mg. The Mg69Zn27Ca4 glassy alloy has the lowest corrosion rate due to its structural and chemical homogeneity and the absence of second crystal phases[9] and [11]. The corrosion rate increases with the increment of Y content, which could be ascribed to the micro-galvanic corrosion caused by the Mg12YZn and Ca2Mg6Zn3 phases. The results of hydrogen evolution are in agreement with the polarization curves (Fig. 6).
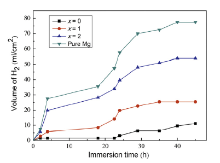 | Fig. 6 Hydrogen evolution volume of Mg69-xZn27Ca4Yx (x = 0, 1, and 2 at.%) alloys and pure Mg in SBF at 37 ° C. |
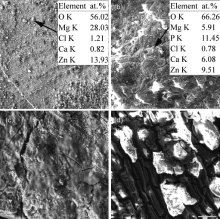
The surface morphologies of the tested materials immersed in SBF at 37 ° C after 85 h are shown in Fig. 7. It is visible that the Mg69Zn27Ca4 alloy maintains the integrity of surface. Although the Y-bearing alloys exhibit a cracked morphology, they are still characterized by a relatively higher integrity compared with pure Mg. The large holes and the small amount of corrosion products indicate that pure Mg is corroded severely mainly through pitting corrosion, and there is not protective film to cover its matrix (Fig. 7(d)). EDS results show that the corrosion products on the matrix of the Mg69Zn27Ca4 alloy are mainly rich in O, Mg, and Zn (indicated by arrow in Fig. 7(a)). Zn2+ will release during the corrosion process resulting in the formation of Zn(OH)2. Zn(OH)2 has lower solubility compared with Mg(OH)2 indicating a better protective effect than Mg(OH)2[9]. This finding suggests that the co-existence of Zn(OH)2 and Mg(OH)2 provides a uniform protective film on Mg69Zn27Ca4 alloy[28]. In the case of Mg68Zn27Ca4Y1 alloy, other elements such as P and Ca are on the matrix (indicated by arrow in Fig. 7(b)). It suggests that Mg and Ca containing phosphates form on the surface of Mg68Zn27Ca4Y1 alloy. Magnesium phosphate (Mg3(PO4)2) compound was reported to form in SBF[29]. The ratio of Ca to P (Ca/P) was approximately 0.53, which was a little lower than 0.75 [Mg3Ca3(PO4)4].
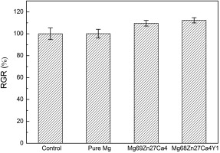
Fig. 8 illustrates the RGR of osteoblasts after 3 days incubation. There is no significant difference between the RGR in pure Mg extracts and that in the negative control. Moreover, the bulk metallic glass composites present higher RGR of cells than the control, indicating a better biocompatibility. According to ISO 10993-5: 2009[30], the cytotoxicity in the extracts of these bulk metallic glass composites was grade 0-1. In addition, Mg68Zn27Ca4Y1 alloy exhibited the highest absorbance.
With the addition of Y, the volume fractions of the crystalline phases increased and the single amorphous phase was destroyed. It means that Y could ruin GFA of Mg-Zn-Ca metallic glass.
The results of SEM and EDS (Fig. 3 and Table 2) show that the snowflake phases are composed of the Mg12YZn and Ca2Mg6Zn3 phases. Ca2Mg6Zn3 is a common strengthening phase in as-cast Mg-Zn-Ca alloy. It is trigonal with the lattice parameters of a = 0.97 nm, c = 1.0 nm [31]. Levi et al. [32] reported that Mg-Zn-Ca alloy could be hardening with the appearance of Ca2Mg6Zn3 phase, which is still stable at a relative low temperature. Mg12YZn phase is very attractive for its special crystal structure (long-period stacking ordered structure) and excellent properties in recent years [33], [34], [35], [36] and [37]. Xiao et al. [34] and Hagihara et al. [35] showed that the Mg12YZn phase could improve the mechanical properties of the Mg88Zn5Y7 alloy, for its elastic modulus is significantly higher than the metallic matrix. Due to the high Young's modulus, Mg12YZn could bear more load transfer during plastic deformation. The two-phase material can be regarded as a composite material, where fine Mg12YZn phase serves as fiber to reinforce the metallic matrix [36]. The excellent comprehensive mechanical properties of Mg68Zn27Ca4Y1 alloy could be attributed to the effective strengthening phases, namely Ca2Mg6Zn3 phase and Mg12YZn phase dispersing on the surface uniformly.
With the addition of 2 at.% Y, the compressive strength decreased. However, the Mg67Zn27Ca4Y2 alloy still exhibited higher strength (770 MPa) than the Mg69Zn27Ca4 glassy alloy (550 MPa) and obvious plastic strain. The decrease in compressive strength could be attributed to the second phases, which became coarse in Mg67Zn27Ca4Y2. The stress concentration could occur due to the larger volume of the second phases. Therefore, the interface between the matrix and the second phases would bear uneven distribution of loading, where the initial cracks would occur[38]. In addition, the Young's modulus of Mg69Zn27Ca4, Mg68Zn27Ca4Y1, and Mg67Zn27Ca4Y2 alloys is 51, 57 and 53 GPa, respectively, which are significantly close to that of the cortical bone (10-40 GPa). Compared with the medical stainless steel (200 GPa), Mg-Zn-Ca-Y alloys could greatly decrease the “ stress shielding” phenomenon.
Numerous reactions took place during magnesium dissolution. These reactions can be simply illustrated as follows[29]:
equation(1)
anodic reaction: Mg → Mg+ + e
equation(2)
Mg+ → Mg2+ + e
equation(3)
cathodic reaction: 2H2O + 2e → 2OH- + H2
equation(4)
chemical reaction (NDE): Mg+ + H2O → Mg2+ + OH- + 1/2H2
equation(5)
formation of corrosion products: Mg2+ + OH- → Mg(OH)2
It is suggested that hydrogen evolution could occur through both the cathodic water reduction and the chemical reaction between water and the dissolved monovalent Mg+ ions. The cathodic current density of the Y-bearing alloys was higher than that of pure Mg in the cathodic region (Fig. 5), which signified that the cathodic reactions (Eq. (3)) were more active for the Y-bearing alloys due to the cathodic effect of Mg12YZn and Ca2Mg6Zn3 phases. Higher cathodic current density implies higher cathodic hydrogen evolution rate of Y-bearing alloys. However, the total hydrogen evolution rate of Y-bearing alloys was lower than that of pure Mg (Fig. 6) because of the anode hydrogen evolution rate (Eq. (4)), which was much lower due to negative difference effect (NDE)[28].
Once corrosion products Mg(OH)2 formed, Cl- as aggressive ions would transform Mg(OH)2 into soluble MgCl2[18]. SBF solution also contained 4PO3-PO43-, Ca2+ and so on. Thus, dissolved Mg2+ would combine 4PO3-PO43- and Ca2+ to form magnesium-containing calcium phosphates according to the following reaction[39]:
equation(6)
-4HPO+Mg2++Ca2++OH-→ xMgyCaz(PO4)HPO4-+Mg2++Ca2++OH-→ MgxCay(PO4)z 
It is true that magnesium-containing calcium phosphates formed on the surface of Mg68Zn27Ca4Y1 alloy rather than on Mg69Zn27Ca alloy according to EDS results (Fig. 7). Researches[39] and [40] showed that magnesium-containing calcium phosphates were better than HA (Ca10(PO4)6(OH)2) in terms of promoting osteoinductivity and osteoconductivity. It can be concluded that the addition of Y facilitated the formation of Mg and Ca containing phosphates, predicting good biocompatibility of Y-bearing alloys.
4.3. BiocompatibilityThe in vitro cytotoxicity of Y-bearing alloys was grade 0-1, indicating that the Y-doped Mg-Zn-Ca metallic glass composites were safe as biomaterials. The cells presented the highest growth rate in Mg68Zn27Ca4Y1 alloy extraction medium. Both Mg2+ and Ca2+ could enhance the cell attachment and proliferation [18]. Gu et al. [25] found that Mg-1Y alloy extracts showed decreased cell viability for L-929, NIH3T3, ECV304 and VSMC cells at concentration of 2.3 ± 0.7 μ mol/L for Y. In our study, Y promoted the formation of magnesium-containing calcium phosphates. Xu et al. [41] reported that calcium phosphate coating significantly improved the surface cytocompatibility of magnesium. Thus, Mg68Zn27Ca4Y1 alloy which exhibited the highest RGR ( Fig. 8) showed good biocompatibility.
The as-cast Mg69-xZn27Ca4Yx (x = 0, 1 and 2 at.%) bulk metallic glass composites were succeeded to be synthesized by Cu-mold injection casting method using industrial raw materials. The Mg68Zn27Ca4Y1 alloy exhibited the highest strength, above 1000 MPa with the plastic strain of 3.1%. All the Y-bearing Mg-Zn-Ca alloys, which had much more noble corrosion potential (Ecorr), lower corrosion current density (icorr) and lower hydrogen evolution rate than pure Mg, displayed good bio-corrosion resistance in SBF solution at 37 ° C. In addition, Y promoted the formation of magnesium-containing calcium phosphates. The presence of magnesium-containing calcium phosphates and in vitro cytotoxicity tests showed the perfect biocompatibility of Mg68Zn27Ca4Y1 alloy. From the above results, the Y-bearing Mg-Zn-Ca alloys, particularly Mg68Zn27Ca4Y1 alloy, exhibiting ultrahigh strength as well as good bio-corrosion resistance and good biocompatibility, could be potential candidate in future biomedical application.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|