Hot ductility, stress-strain behavior and high temperature tensile fracture behavior of wrought 316LN stainless steel were investigated. Hot tensile tests were carried out on a Gleeble 1500D thermal simulator system at a strain rate of 0.5 s-1 over the temperature range 650-1300℃. The percentage reduction of area (RA) decreased with the increasing deformation temperature over the range of 650-850℃, and then starting from 850℃, it began to increase dramatically with values over 85% above 1000℃. When the deformation temperature comes to 1300℃, RA decreased sharply as a result of the grain coarsening due to over-heating. With the help of optical microscopy, dynamic recrystallization (DRX) was observed for the steel deformed at temperature over 1000℃. The enhancement of ductility induced by DRX was considered to play an important role in inhibition of the crack propagation. The high temperature tensile failure process of 316LN includes the nucleation, growth, and aggregation of microscopic cavities. The SEM/EDS results show that the sulfide and alumina at grain boundaries may be responsible to the formation process of cracks.
Aluminum alloy 7075 (Al-Zn-Mg-Cu) is attractive for a number of structural applications owing to its high strength to weight ratio and natural aging characteristics[1] and [2]. The choice of welding methods for its further applications are important. Friction stir welding (FSW) is a new and potential weld method in industry, and it strongly reduces distortion and residual stresses compared to fusion welding techniques[3] and [4]. However, the major problem for the 7075 aluminum alloy in FSW is the weld joints exhibiting corrosion susceptibility[5]. Having recognized this as one of the major concerns in FSW of high-strength 7075 aluminum alloys, a few attempts have recently been made to overcome the problem using post-weld heat treatments, laser surface treatments and other approaches[6], [7], [8], [9] and [10]. Nonetheless, the durability of the corrosion protection by these methods might be short-term.
Micro-arc oxidation (MAO) is a novel technique that can be used to form a thick ceramic coating on aluminum substrate by plasma discharge in aqueous solution on aluminum surface at high voltage[11], [12] and [13]. Furthermore, the coating has been proved as an effective method to improve the corrosion resistance of aluminum FSW joints[14]. It is known from our previous study[15] that, electrolyte composition is one of the most important parameters affecting microstructure and properties of the coating in the process of MAO. In the electrolyte, SO42-, Cl-, SiO32- and CO32- show different impedance values, while SiO32- shows that the highest impedance value of the film is 36, 005 Ω . It is believed that the formation of a compact coating with high impedance value on the sample surface is primary; hence, Na2SiO3 is chosen as the main contents of the electrolyte in the present study[16]. The final objective in this study is to present routes for the optimization of MAO electrolyte concentration, and thus the effect of Na2SiO3 concentration in electrolyte on the corrosion resistance of Al2O3 coatings formed on friction stir welding joint was analyzed.
The morphology of the welds is shown in Fig. 1. It is seen that the welding nuclear zone and heat affected zone with fine microstructure were obviously formed on it. Samples cut from 7075 aluminum alloy FSW welds with dimensions of 40 mm × 60 mm × 7 mm were used as substrates for micro-arc oxidation. MAO units used in this work mainly consist of a power source with approximate 1000 V and an electrolyte consisting of Na2SiO3 10 g/L, 15 g/L, 20 g/L and NaOH 5 g/L, current density was kept at less than 5 A/dm2. During the coating process, the temperature of the electrolyte was maintained at approximately 30 ° C controlled by a heat exchanger. All the samples were oxidized for approximately 40 min. icrostructural observation was carried out by scanning electron microscopy (SEM, JSM-5600, Japan). The phase composition of the coatings was identified by X-ray diffraction (XRD, Model D/Max 2500PC Rigaku, Japan) operated with Cu Kα . The X-ray generator settings were 50 kV and 50 mA, and the scans were acquired from 30° to 90° (in 2θ ). The 3D microstructure and the coatings thickness were observed by confocal laser scanning microscopy (CLSM, Olympus OLS3000, Japan).
The corrosion behaviors of the coatings prepared at different electrolyte concentration were evaluated by electrochemical impedance spectroscopy (EIS) and potentio-dynamic polarization test through an AUTO-PGSTAT 302 electrochemical analyzer in 3.5% NaCl solution at room temperature. The electrochemical measurement was conducted using a conventional three electrodes system with a saturated calomel electrode (SCE) as the reference, a Pt foil as the counter electrode, and the samples with the area of 1 cm2 as the working electrode. For EIS study, AC impedance measurements were performed with the amplitude of 10 mV about the open-circuit potential versus frequency from 10 mHz to 1 MHz. The polarization scan rate was controlled at 0.2 mV/s. The electrochemical parameters of corrosion potential, corrosion current density and polarization resistance were analyzed.
Fig. 2 shows the top morphologies of the coatings formed in the electrolyte with different Na2SiO3 concentrations. All the coatings were compact, the crack-like structure and the small pores were observed. MAO coatings are known to exhibit some degree of porosity and micro-cracks, especially towards the top surface[15]. In the present study, some small residual pores can also be seen on the surface of the coatings. These pores are formed by the molten oxide and gas bubbles thrown out of micro-arc discharge channels. Besides, the microcracks generated from the thermal stress in fast solidification of melts also existed near the pores. As increasing the Na2SiO3 concentration from 10 g/L to 20 g/L, the coatings become denser, and the plasma discharging in aqueous solution on the surface of aluminum substrate at high voltage seems to be intensive. On the other side, the diameter of the pores apparently decreased with increasing Na2SiO3 concentration in electrolyte. The changes of the microstructure can be explained by the electrical conductivity of the electrolytes. The electrical conductivity of the electrolytes increases with increasing the Na2SiO3 concentration. The higher Na2SiO3 concentration corresponds to a higher current and thus a more intensive micro-arc discharge will occur on the surface. However, the obtained microstructure was quite different from that in our previous study[15]. This is attributed to that the samples in the present study experienced the FSW process making the original microstructure of as-received aluminum alloy changed. Fig. 3 displays the phase compositions of the alumina coatings on FSW joints in electrolyte with different Na2SiO3 concentrations. It can be found that all the coatings are mainly composed of Al2O3. There is no obvious difference from the XRD results.
 | Fig. 2 Surface morphologies of the MAO coatings formed on the welds in electrolyte with different Na2SiO3 concentrations: (a) 10 g/L; (b) 15 g/L; (c) 20 g/L. |
Fig. 4 illustrates the two-dimension and three-dimension images of the coatings formed on 7075 aluminum alloy welds in the bath with different Na2SiO3 concentrations for 40 min. The micrographs clearly indicate that the pores presenting as dark circular spots distributed all over the surface of the ceramic coatings. It is also apparent that the number of pores increases, while the diameter of the pores obviously decreases with increasing the Na2SiO3 concentration in electrolyte, just as shown in the insets of Fig. 4. The results for coating thickness measured by the software attached to the CLSM are presented in Table 1. It can be seen that the thickness of ceramic coatings produced on friction stir welds was about 23-35 μ m by micro-arc oxidation treatment. Though all the coatings exhibit the similar surface morphologies, the wave crest and trough of the agglomeration varies depending on the electrolyte concentration. It clearly shows that the surface roughness decreases with increasing Na2SiO3 concentration. The changes of surface morphologies could be exclusively contributed to the effect of Na2SiO3 concentration in electrolyte which determined the electrical conductivity in the MAO process. Therefore, the higher the Na2SiO3 concentration in electrolyte is, the smaller the dimension of discharged channel and the lower the surface roughness in surface appearance will be. However, once the employed electrolyte concentration was higher, the intensive discharging could be observed, which is harmful to the quality of the coating. Therefore, the Na2SiO3 concentration of 10-20 g/L is suitable in the present work.
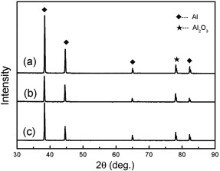 | Fig. 3 XRD results of the coatings formed in electrolyte with different Na2SiO3 concentrations: (a) 10 g/L; (b) 15 g/L; (c) 20 g/L. |
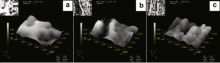 | Fig. 4 3D images of MAO coatings formed on the welds in electrolyte with different Na2SiO3 concentrations: (a) 10 g/L; (b) 15 g/L; (c) 20 g/L. |
| Table 1 Thickness of MAO coatings formed in electrolyte with different Na2SiO3 concentrations |
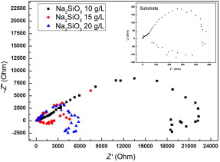
Electrolyte concentration influenced the morphology of the coatings, and therefore, it is bound to influence the corrosion resistance of the coatings. In the present work, EIS and potentiodynamic polarization were used to evaluate the corrosion resistance of the FSW welds with and without MAO coatings in 3.5 wt% NaCl solutions. The EIS plots and the potentiodynamic polarization plots are presented in Fig. 5 and Fig. 6, respectively. From Fig. 5, the corrosion resistance of the samples with micro-arc oxidation coatings increased obviously compared to as-received welds. The corrosion potential (Ecorr) of the coatings produced with 20 g/L Na2SiO3 in electrolyte is much higher than the other two. As for the corrosion current density (icorr), the samples with Al2O3 coatings exhibited lower corrosion current density than the as-received FSW weld. Furthermore, it should be noted that the corrosion current density of the coatings produced with 20 g/L Na2SiO3 in electrolyte is far lower than those coatings produced at 10 g/L and 15 g/L Na2SiO3. On the other hand, for the EIS results, there is only one loop for all the coatings which indicates the existence of only one time constant in the frequency by a simple equivalent circuit consisting of a resistance Rp and a constant phase element Q in parallel appeared in Fig. 6. The value of Rp, the resistance of the MAO layer, has an increase of two orders of magnitude compared with the as-received FSW welds just as shown in the inset of Fig. 6. As described above, the corrosion resistance of the FSW welds with ceramic coatings greatly improved when it was fabricated in electrolyte with 20 g/L Na2SiO3. It is well known that the anti-corrosion efficiency of the coatings is strongly dependent on the microstructure and phases, coating thickness, etc. Pitting corrosion is the main corrosion form of alumina coating in NaCl solution. Therefore, denser and finer microstructure with smaller pores and thicker coating is helpful to improve the corrosion resistance by inhibiting the attack from the Cl- in the NaCl solution. Improved corrosion resistance of MAO-treated welds can be attributed to the presence of highly corrosion-resistant oxides on weld surfaces. Overall, the results clearly confirm that MAO coatings are highly effective in improving the corrosion resistance of friction stir welds of 7075 aluminum alloy. And, whether the MAO coatings formed on the weld have any other effects on the mechanical properties of FSW joint will be conducted in other studies.
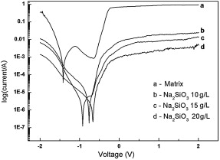 | Fig. 5 Potentiodynamic polarization curves for substrate and MAO coatings produced in electrolyte with different Na2SiO3 concentrations. |
The ceramic coatings were successfully produced on friction stir welds of 7075 aluminum alloy by micro-arc oxidation. The Na2SiO3 concentration in electrolyte considerably affects the morphologies, thickness and corrosion resistance of the ceramic coatings on welds. Electrochemical results showed that substantial improvement in corrosion resistance of the friction stir welds can be achieved by MAO treatment. Furthermore, the ceramic coating formed at 20 g/L Na2SiO3 in electrolyte demonstrates the superior corrosion resistance than the others.
This work was supported by the Program for New Century Excellent Talents from the Ministry of Education (Grant No. NCET-11-0984).
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|