Oxidation Behaviors of C-ZrB2-SiC Composite at 2100 °C in Air and O2
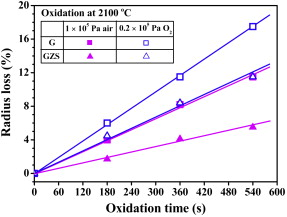
Fig. 1. Oxidation kinetics of G and GZS at 2100 #cod#x000b0;C in 1 #cod#x000D7; 105 Pa air and 0.2 #cod#x000D7; 105 Pa O2.
Oxidation Behaviors of C-ZrB2-SiC Composite at 2100 °C in Air and O2 |
| Fig. 1 shows the curves of radius loss vs oxidation time for G and GZS at 2100 #cod#x000b0;C in 1 #cod#x000D7; 10 5 Pa air and 0.2 #cod#x000D7; 10 5 Pa O 2 . It can be seen that, in all conditions, the radius loss of both graphite and the graphite-based composite increases linearly with the increase of the oxidation time. Gao et al. [ 15 ] have investigated the effect of the incorporation of ZrB 2 and SiC into graphite on its oxidation resistance at 1600 #cod#x000b0;C in air. Linear rate law was also obtained in their work. Besides, their results have shown that the additions of these ceramic particles can decrease greatly the radius loss of graphite during oxidation at 1600 #cod#x000b0;C. In the present work, GZS also displays much lower radius losses than G during oxidation in the same atmospheres at 2100 #cod#x000b0;C see Fig. 1 . It indicates that the improvement of the oxidation resistance of graphite by adding ZrB 2 and SiC still works at least up to 2100 #cod#x000b0;C. More interestingly, however, both G and GZS experienced different oxidation behaviors during oxidation in 0.2 #cod#x000D7; 10 5 Pa O 2 and in 1 #cod#x000D7; 10 5 Pa air, Although the same oxygen partial pressure of 0.2 #cod#x000D7; 10 5 Pa was maintained in the testing chamber, the oxidation of G and GZS in 0.2 #cod#x000D7; 10 5 Pa O 2 resulted in more severe radius losses than that in 1 #cod#x000D7; 10 5 Pa air. It suggests that the total gas pressure in the testing chamber also have a significant impact on the oxidation behaviors of G and GZS. Fig. 1. Oxidation kinetics of G and GZS at 2100 #cod#x000b0;C in 1 #cod#x000D7; 105 Pa air and 0.2 #cod#x000D7; 105 Pa O2. |
 |