Coal fly ash is an industrial by-product generated during the combustion of coal for energy production. Due to the increasing annual consumption of coal power and the serious potential environmental threats of coal fly ash, a considerable amount of research on the utilization of coal fly ash has been undertaken worldwide. Vitrification seems to be one of the most promising options for reusing this industrial waste. This paper presents a short overview of the production of unique high performance glass-ceramics using coal fly ash as a raw material. A detailed description of the methodologies for the synthesis of glass-ceramics from coal fly ash and the principal crystal phases, corresponding property and possible usage of those materials are introduced. Investigations revealed that converting coal fly ash into high performance glass-ceramic materials is a promising new approach to improve the utilization of this industrial by-product. This conversion not only alleviates the problems with disposal but also converts a waste material into a high value-added marketable commodity.
Coal is currently a significant source of fuel worldwide. Coal-fired power plants are in most countries the main source of electrical energy, accounting for about 40% of the worldwide electricity production. Coal fly ash, generated during the combustion of coal, is an industrial by-product whose current annual production is estimated to be more than 500 million tons[1], a number that continues to increase to account for the growth in power demand. This large amount industrial waste can cause significant environmental and ecological problems if not treated properly. Research has been undertaken worldwide on the potential reuse and effective utilization of this waste due to its ecologic and economic importance. In recent years, many investigations on the utilization of fly ash for glass-ceramics production have been reported[2], [3], [4], [5] and [6]. The results are encouraging for it was found that the toxic trace elements were completely destroyed and the heavy metals were successfully solidified into the glass matrix during the process of high temperature vitrification and the consequent nucleation and crystallization heat-treatments[7] and [8]. Therefore, the glass-ceramics produced from coal fly ash are non-hazardous, valuable materials showing promising potential applications.
From the perspective of power generation, coal fly ash is a waste by-product, while from a coal utilization point it is a resource yet to be fully utilized. As a fine-grained powder, fly ash is mainly composed of spherical glassy particles that contain valuable mineral resources such as SiO2, Al2O3, CaO, Fe2O3 and other oxides. The chemical composition and fine-grained powder morphology of fly ash makes it suitable for use as the raw material for glass-ceramics, a valuable material that is not only suitable for replacing many traditional materials, but is also useful in entirely new fields where no alternative material can satisfy the technical demands[9].
In this paper this promising new approach to utilizing fly ash was reviewed with an emphasis on the new, high infrared radiance glass-ceramics produced from coal fly ash. In addition, a new area or field that could expand the positive reuse of fly ash was sought, thereby seeking to reduce the environmental and economic impacts of disposal. The results of this investigation are not strictly confined to coal fly ash, and the fly ashes of other fuels, such as oil shale[10] and municipal solid waste incinerator fly ashes, may be utilized as raw materials for this glass-ceramics production[11], [12] and [13].
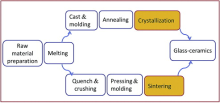
There are two traditional methods to produce glass-ceramics - the crystallization approach, and the sintering approach, both of which are shown in Fig. 1[14] and [15].
Generally, regardless of which approach is pursued, the properties of glass-ceramics are determined by the main crystallization phases and their microstructures, which depend on the composition of the parent glass as well as the thermal treatments. It is therefore important to design the composition and time-temperature schedules to achieve the desired crystal phase, microstructure, and corresponding properties[16].
It is difficult to synthesize glass-ceramics from fly ash directly without any additives, for in most conditions, the fly ash does not contain proper ratios of components for the formation of a glass. The addition of glass network modifiers is needed to fully achieve vitrification of fly ash. Na2O is the most effective glass modifier, as it can decrease the melting temperature and melting viscosity of the parent glass and make the processing operations easier. However the addition of Na2O can also decrease the chemical durability and compressive strength of the obtained glass[17] and [18]. B2O3 are network-forming elements in glass structure, and a proper amount of B2O3 in the glass matrix can promote nucleation of the glass[19]. Aside from addition of glass network modifiers, the main contents, such as CaO and MgO, and nucleating agents, such as TiO2 and ZrO2 are typically also required. Trace contents such as V2O5 and BaO also have great influences on the processing operations and the final properties of the fly ash glass-ceramics[20], [21], [22], [23] and [24].
According to the American Society for Testing Materials[25], the ash containing more than 70 wt% SiO2 + Al2O3 + Fe2O3 that is also low in lime is defined as class F fly ash, while ash with a SiO2 + Al2O3 + Fe2O3 content between 50 and 70 wt% that is high in lime is defined as class C fly ash. The chief difference between Class F and Class C fly ash is in the amount of calcium and the silica, alumina, and iron content in the ash. Due to the varying chemical compositions of different classes of coal fly ash, normally there are two main categories of coal fly ash glass-ceramics[26], CaO-Al2O3-SiO2 (CAS) system coal fly ash glass-ceramics and MgO-Al2O3-SiO2 (MAS) system coal fly ash glass-ceramics.
2.1.1. CaO-Al2O3-SiO2 system coal fly ash glass-ceramics
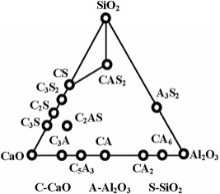
Class C coal fly ash is much more suitable for synthesizing CaO-Al2O3-SiO2 system glass-ceramics. This system glass-ceramics have many excellent properties such as high mechanical strength, excellent dimensional stability, and abrasion, corrosion resistance, demonstrating potential for a wide range of application on construction. The main phases of this system glass-ceramics usually include anorthite (CaAl2Si2O8), wollastonite (CaSiO3), gehlenite (Ca2Al2SiO7) and diopside (CaMgSi2O6). The various phases are related to the differences in composition (Fig. 2)[27].
To synthesize CAS system glass-ceramics from coal fly ash, addition of CaO is required during the composition design process. Some researchers reported that the addition of CaO can be accomplished by using shell and other CaO-rich materials as raw materials, which can decrease the cost of final glass-ceramics production further[28]. The ratio of CaO/SiO2 can influence the processing operations and the final properties of the glass-ceramics decisively[29]. Experimental results show that an increase of CaO amount can decrease the crystallization activation energies and promote the crystallization of glass-ceramics. However, when there is an increase of CaO content, the range of the sintering temperature is decreased, which in this case has a negative effect on the sintering, leveling and compacting processes[30].
2.1.2. MgO-Al2O3-SiO2 system coal fly ash glass-ceramics
Differing from class C coal fly ash, class F coal fly ash is not suitable for the production of CAS glass-ceramics and cement additives due to its relatively high MgO and Fe2O3 content. However the class F coal fly ash can be used to synthesize MgO-Al2O3-SiO2 system coal fly ash glass-ceramics[31], [32], [33] and [34], whose main phases are usually cordierite (2MgO· 2Al2O3· 5SiO2) or mullite (2Al2O3· SiO2). Due to its many outstanding properties such as an elevated thermal stability, good chemical durability, and very low thermal expansion coefficient, this system glass-ceramics is widely used, especially in the high temperature field where a excellent thermal shock resistance is required[35]. Recently, research has been performed on the high infrared radiance MAS system glass-ceramics made from class F coal fly ash and slags. The main phase of these high infrared radiation glass-ceramics is a heterogeneous ion incorporated solid solution ((Mg, Fe)2Al4Si5O18)[36]. Because the crystal structures of cordierite are relatively loose, the Mg-O, Al-O bond connecting the [SiO4] tetrahedral and the [AlO4] tetrahedral is not very close. Along the crystal C-axis direction, there is a greater access gap, which can accommodate other doping ions[37]. When fabricating glass-ceramics with class F coal fly ash which containing iron oxide content, Iron ions and other impurity ions will incorporate into the cordierite structure by means of substitutional diffusion, where Fe3+ substitute Mg2+ forming vacancy defects[38]. The vacancy defect-reaction equation can be written as:
equation(1)
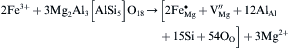

Where
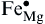
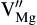
OOOO— The in-situ oxygen.
The defect chemical formula of Fe3+ substituted Mg2+ in the cordierite crystal structure is as follows:
equation(2)
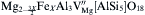

The formation of Mg2+ vacancies partially damaged the symmetry of the lattice and caused lattice distortion, leading to changes in molecular vibration and rotational states. In addition, the formation of impurity energy levels in the local area increased the transition probability of electrons from the full to the conduction band. So the mechanisms of high infrared radiation concern three main aspects: the impurity effect, the vacancy defect effects, and the lattice distortion effect. High infrared radiance (whole band normal emissivity more than 0.90 and thermal expansion coefficient of (1-2) × 10-6 K-1 between 20 and 800 ° C) glass-ceramics obtained from fly ash can be used as infrared heating and drying materials[36], [38] and [37]Table 1.
| Table 1 Infrared radiance of fly ash glass-ceramic, 4% Fe2O3-doped and non-doped glass-ceramic samples (test performed at 70 ° C). F1 signifies the whole-band normal direction radiance, F2 signifies the radiance at the 2-5 μ m waveband, and F3 signifies the radiance at the 8-15 μ m waveband |
To obtain high performance fly ash glass-ceramics, it is necessary to control the crystallization behaviors of the obtained parent glass, namely, to determine the optimum nucleation and crystal growth temperatures and duration. Normally, Differential Thermal Analysis (DTA) is used to determine the glass transition temperature (Tg) and the exothermic crystallization peak (Tp). The optimum nucleation temperature is usually 50-100 ° C above glass transition temperature [39] and [40]. The crystallization peak temperature was selected to be the optimum crystal growth temperature. Generally, the nucleation and crystallization temperatures are slightly lower than those of a conventional reagent glass-ceramics of the same contents. According to the experiment result of DTA, a plot of the height of the exothermic crystallization peaks (δ Tp) vs the pre-heated temperatures (50-100 ° C above Tg) can be obtained. The height of the exothermic crystallization peaks reaches a plateau after a duration of heat treatment. The optimum nucleation time was selected as this duration. The optimum duration of heat treatment for crystallization can be determined in the same manner as the nucleation time, and it can also be determined from the maximum in the plot of the intensity of the highest XRD peak (In) versus the total intensity of peaks in the XRD patterns (Itotal) for different amounts of time [41].
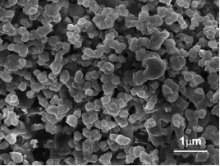
Many investigations revealed that the coal fly ash glass-ceramics often demonstrate the microstructure of superfine or nanoscale spherical crystals homogeneously dispersed within the parent glass matrix as shown in Fig. 3[36] and [42]. Due to their individual microstructures, this fine-grained glass-ceramics often possess improved properties such as increased hardness, strength[40] and [43]. Aside from the main contents of SiO2, Al2O3, Fe2O3, and CaO, fly ash also contains different minor, but essential, oxides of these elements, such as Cu, Mn, B, Mo, Ti, Na, K, and Mg. These oxides can play important roles in the processing procedures and the properties of the final fly ash glass-ceramics products, such as by acting as composite nucleating agents in the process of nucleation of the parent glass obtained from ash, as coloring agents in the formation of vivid colors, and as solid solution dopants in the improvement of the electrical, optical, and magnetic properties of the glass-ceramics materials. A presumption of the nanostructure of the fly ash glass-ceramics is that phase separation occurs before the nucleation of the fly ash vitrified glasses due to the minor content of oxides, which act as composite nucleating agents, as shown in Fig. 4[38]. The interfaces of the two phases provide the core of the heterogeneous nucleation for the precipitation of the consequent crystalline, which thereby reduces the crystallization activation energy, promoting its nucleating[44] and [45]. The formation of large amounts of crystal nucleus makes the consequent grain growth difficult. Therefore, comparing to the traditional glass-ceramics synthesized from pure raw materials, fly ash glass-ceramics are more apt to form nano-crystal microstructure[46].
Glass-ceramics derived from coal fly ash can have distinctive chemical properties and attractive physical appearances similar to or even superior to those of natural granites and marbles[47] and [48]. There are many potential applications, such as wall-covering panels, floors and roofs in industrial and public buildings, interior facings of containers, and road surfacing. These ceramics are predicted to have service lives between 20 and 45 years provided they are not exposed to intensive impact.
On account of the possibilities for energy conservation and reduction of pollution, infrared heating and drying technology is being used more frequently in the industry[49] and [50]. As a consequence, there is an increased demand for high infrared radiance materials. Well-crystallized (bulk crystallization, granular equiaxed grains, sized 200-400 nm) iron ion (Fe2+ and Fe3+) substituted cordierite-based glass-ceramics, obtained from class F coal fly ash upon the addition of the proper amount of MgO, have excellent infrared radiation performance (the whole-band normal direction radiance is about 0.93-0.94), low thermal expansion coefficients ((18-21) × 10-7 K-1), and good thermal shock resistance, These properties result in the glass-ceramics derived from class F coal ash being suitable for application in a wide range of infrared heating and drying materials[36] and [38]. In addition, there are several reports on the preparation of low-cost cordierite-based porous ceramic membranes from this industrial waste[51] and [52]. Fly ash glass-ceramic membranes and coatings with good infrared radiation performance can also be obtained. In this case, the thermal expansion coefficient of the glass-ceramics can be adjusted by controlling the ratio of the main phases.
It is demonstrated that producing glass-ceramics is promising and valuable by utilizing coal fly ash. The synthesis of glass-ceramics from fly ash is not only low cost, but the product also has many advantageous properties such as being more apt to crystallization (lower crystallization temperature and shorter crystallization time) and the formation of nanocrystals (fine microstructure), where the formation of nano-crystals is beneficial to mechanical (and other functional) properties. Impurity effects of trace elements are beneficial to infrared radiation properties. Although much work must be done to achieve the commercial production of fly ash glass-ceramics, the ecologic and economic importance of reducing the coal ash waste may be sufficient to stimulate the necessary research.