Dual-phase membranes of 60 wt% Ce0.8Gd0.2O2- δ-40 wt% PrBaCo2 -xFe xO3- δ (0 ≤ x ≤ 2) were prepared by combined citrate and ethylene diamine tetraacetic acid (EDTA) complexing method. X-ray diffraction (XRD) results revealed the good chemical compatibility between ion-conducting phase CGO and electron-conducting phases PBC2 -xF xO after sintering in air. The Fe ionic dopant had a significant effect on the phase structure stability and oxygen permeability under CO2 atmosphere, which was confirmed by XRD, thermogravimetry-differential scanning calorimetry (TG-DSC), scanning electron microscopy (SEM) and oxygen permeation experiments. CGO-PBC0.5F1.5O dual-phase membrane demonstrated a stable oxygen permeation flux of 2.71 × 10-7 mol cm-2 s-1 with 50 mol% He/CO2 as the sweep gas at 925 °C, and this value was much higher than that of perovskite-type membranes. The rate-limiting step in the oxygen permeation process changed from the bulk diffusion to the surface oxygen exchange when the CGO-PBC0.5F1.5O membrane thickness decreased to 0.8 mm or less. Due to the high oxygen permeation fluxes and the excellent structural stability under CO2 atmosphere, the CGO-PBC0.5F1.5O membrane is a great potential candidate material for separating oxygen from air in the oxy-fuel combustion techniques.
Mixed oxygen ionic and electronic conducting membranes have attracted considerable attention due to their potential application in oxygen separation from air with infinite selectivity under different oxygen partial pressure gradients and oxy-fuel combustion technique for CO2 capture[1], [2] and [3]. A large number of studies on single phase membrane of perovskite structure have been devoted to obtain excellent oxygen permeability, such as SrCo0.8Fe0.2O3-δ , BaCo0.7Fe0.2Nb0.1O3-δ and Ba0.5Sr0.5Co0.8Fe0.2O3-δ [4], [5], [6] and [7]. These materials are simply designed, low manufacturing cost and high oxygen permeation. Unfortunately, the perovskite materials are very sensitive to CO2 because the alkaline-earth elements on the A-site can easily react with CO2 or SO2 to form the carbonates and sulfates [8]. Yang et al. [9] found that La0.1Sr0.9Co0.5Fe0.5O3-δ decomposed to carbonate and metallic oxide after sweeping by using CO2. Alkaline-earth-metal-free perovskite-type membranes are generally more durable, but they obtain low permeation flux and still suffer from decomposition in acidic or reducing atmosphere [10] and [11].
Dual-phase composite oxides as mixed conductor have drawn much attention in recent years[12], [13], [14] and [15], as oxygen ions and electrons could be transported through different phases without any interference in the membranes. In a dual-phase composite membrane, the compositions of oxygen ionic conducting phase and electronic conducting phase can be adjusted according to different practical requirements. The fluorite structure oxides have good oxygen ion conductivity and mechanical strength, which was used as the oxygen ion conducting phase, could reduce the thermal expansion coefficient of the dual-phase membrane and increase the chemical compatibility between two phases[16] and [17]. Chen et al.[18] researched the dual-phase membrane made of a noble metal and an oxide ionic conductor zirconia palladium (Pd-YSZ). However, the disadvantages of this kind of cermet membranes were their poor oxygen permeation ability and high cost, which limited their practical application for oxygen separation and oxy-fuel combustion. To overcome these disadvantages, perovskite-type mixed conductor oxides are suggested as good substitutes for noble metal due to their high electronic conductivity and low cost. Some good electron conducting oxides such as La0.8Sr0.2CrO3-δ and La0.7Sr0.3MnO3-δ have been reported to be used as electron conducting phases and Ce0.8Gd0.2O2-δ or Ce0.8Sm0.2O2-δ as oxygen ion conducting phase to form dual-phase composite membranes[19] and [20]. These membranes show considerable oxygen permeation performance as expected, but the chemical compatibility of the electron conducting oxides with the ion conducting oxides and the long term stability of these membranes need to be further improved.
LnBaCo2O5+δ (Ln = rare earth element), double-perovskite oxides, have attracted much attention due to their high oxygen surface exchange coefficients, high electronic conductivity and rapid oxygen-ion diffusion[21], [22] and [23]. The standard structure of LnBaCo2O5+δ has layers of BaO, CoO2 and LnOδ aligned along the c-axis with oxygen vacancies as cluster in the LnOδ layer [24]. Among these materials, the oxygen surface exchange coefficients and oxygen-ion diffusion of PrBaCo2O5+δ (PBCO) oxide are obviously superior to other LnBaCo2O5+δ oxides. Zhang et al. [25] demonstrated that this membrane had an excellent electrical conductivity, reaching as high as 900 S cm-1 at 500 ° C. However, the oxygen permeation flux was significantly lower than that of single-phase perovskite membrane, which cannot meet the industrial application requirements. Our group reported that the introduction of fluorite structure oxide Ce0.8Sm0.2O2-δ (SDC) into the PrBaCo2O5+δ oxide can improve the oxygen permeability and structural stability of membrane because the 3D diffusion ability of SDC can shorten the tortuosity of the oxygen diffusion path in layered PBCO. The maximum oxygen permeation flux is 2.38 × 10-7 mol cm-2 s-1 at 925 ° C with 0.6 mm thickness SDC-PBCO (6/4 vol.%) membrane under He atmosphere [26]. However, the chemical stability and oxygen permeability of CGO-PBCO dual-phase membrane should be further improved. In previous work, we found that the replacement of Co3+ ion by Fe3+ in the double-perovskite structure oxide GdBaCo2O5+δ could have a great impact on their mixed ionic and electronic conducting (MIEC) properties [27].
In this paper, we present the efficacious structure stabilization effect of Fe on dual-phase membrane Ce0.8Gd0.2O2-δ -PrBaCo2O5+δ by doping in the electronic conducting phase PrBaCo2O5+δ , and develop the Ce0.8Gd0.2O2-δ -PrBaCo2-xFexO3-δ (CGO-PBC2-xFxO, 0 ≤ x ≤ 2) system materials, which show considerable oxygen permeability and excellent phase stability under CO2 atmosphere.
The powder of CGO was synthesized by a combined citrate and ethylene diamine tetraacetic acid (EDTA) complexing method. Appropriate amounts of Ce(NO3)2 and Gd2O3 powders (analytical reagent, Sinopharm Chemical Reagent Co., Ltd.) were respectively dissolved in distilled water and a dilute nitric acid solution. These two solutions were mixed in the desired cation stoichiometry, calculated amounts of citrate and EDTA were added and the pH value was adjusted to 6-8 by ammonia. The molar ratio of EDTA: citric acid: total metal ions was 1: 1.5: 1. After stirring the solutions for evaporating the water at 90 ° C on a hot plate, the gels were calcined in air at 350 ° C in a muffle furnace to remove the organic compounds by combustions and these powders were calcined at 650 ° C for 5 h. Similar routes were used to synthesize the PrBaCo2-xFexO3-δ (PBC2-xFexO) powders. Appropriate amounts of Pr(NO3)3, Ba(NO3)2, Co(NO3)2· 6H2O and Fe(NO3)3· 6H2O were dissolved in distilled water. These obtained powders were calcined at 850 ° C for 5 h.
The as-obtained CGO and PBC2-xFxO powders were mixed with a weight ratio of 60: 40 in a mortar. After grinding in an agate mortar for 3-4 h carefully, the mixed powders were uniaxially pressed to disk-shape membrane under a pressure of 135-150 MPa in a stainless steel module with a diameter of 20 mm. Then, these green disks (2 g weight per disk) were sintered at 1150-1180 ° C for 8-10 h with heating and cooling rates of 3 ° C min-1. All the dual-phase membranes which were used for oxygen permeation studies had the relative densities higher than 95%.
The phase structure of the as-prepared powders or sintered disks before and after oxygen permeation were characterized by X-ray diffraction (XRD) using Rigaku D/MAX2550 diffractometer in range of 2θ ( CuKα l, λ = 0.154056 nm) from 10° to 90° with a scan step of 0.02° . To investigate the CO2 tolerance of the samples, the samples were heated from 30 to 1100 ° C at a heating rate of 10 ° C min-1 in different CO2 concentration atmospheres in the thermogravimetry-differential scanning calorimetry (TG-DSC) experiment (NETZSCH STA 449 F3). The surface and cross section morphology of the membranes were observed by scanning electron microscopy (SEM) using JSM-6700.
The oxygen permeation fluxes through the membranes were assessed using a home-made equipment. The as-polished membranes were clamped between one silica tube and alumina tube with silver rings as sealing between the sample and the silica tube. The set-up was initially heated in an air/helium or air/carbon dioxide gradient up to 945-948 ° C in order to soften the sliver ring and tighten the sealing. And then, temperature was decreased to the measured temperature and no nitrogen leakage was detected during the measurements. Dried air was fed to the air side at a flow rate of 300 ml min-1 and He/CO2 was fed to the other side. The permeation fluxes were calculated by using the composition of the outlet gas from the permeation side, which was analyzed by using an online gas chromatograph (GC9160).
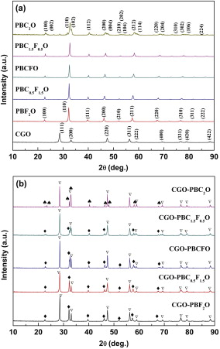
Fig. 1 (a) shows the XRD patterns of the PBC2-xFxO (0 ≤ x ≤ 2) oxides prepared by EDTA-citrate method after calcination at 950 ° C for 5 h under an air atmosphere. For x = 0, the XRD patterns of the PBC2-xFxO powders were identified as double-perovskites and indexed in the basis of a tetragonal structure [27]. It can be seen that the main peaks of PBC2-xFxO (JCPDS No. 53-0131) shift gradually toward the low-angle direction with increasing doping content of larger ion radii Fe3+ in Co-sites. The crystal structure changes from tetragonal to cubic when x ≥ 0.5, indicating a lack of long-range ordering in the perovskite lattice with Fe3+ doping. Fig. 1(b) shows the XRD patterns of composite membranes CGO-PBC2-xFxO. The characteristic peaks are corresponding to the fluorite phase CGO (JPDS No. 46-0507) and the other phases PBC2-xFxO are obvious, and no other peaks resulting from chemical reaction phase between CGO and PBC2-xFxO can be observed, suggesting good chemical compatibility between two phases. The lattice constants of the CGO phase in the dual-phase membranes CGO-PBC2-xFxO do not change, as shown in Table 1, revealing that no cation diffusion happens between CGO and PBC2-xFxO phases. The two phases can maintain their own phase structure in the dual-phase membranes due to their different crystal structures.
| Table 1 Lattice constant and volume of CGO in the dual-phase membranes CGO-PBC2-xFxO |
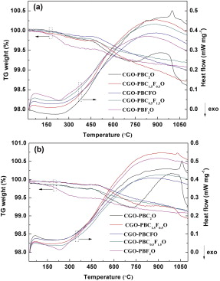
Fig. 2(a) and (b) presents the TG-DSC results of the CGO-PBC2-xFxO powders in 20 mol% and 50 mol% CO2/N2 atmosphere, respectively. It can be seen that all the samples experience a weight loss process with increasing temperature corresponding to desorption of lattice oxygen. Compared to the other samples, sample with CGO-PBC2O exhibits a weight increment and loss in the 20 mol% CO2/N2 atmosphere at high temperature, which may be related to the interaction between CO2 and PBC2O oxide to produce carbonates according the similar research conclusions in the single perovskite membranes[28]. The TG curves of CGO-PBC1.5F0.5O powders show the similar phenomenon with increasing CO2 content to 50%, but it shows slighter weight gain and loss than the CGO-PBC2O sample. The results of TG-DSC experiments indicate that the chemical stability against CO2 is strengthened with increasing Fe concentration in CGO-PBC2-xFxO.
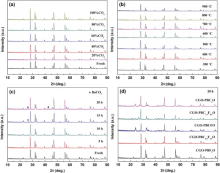
The good stability under CO2-rich atmosphere of dual-phase membranes in long time is a key factor for applying to the oxy-fuel combustion successfully[29]. Therefore, the high-temperature phase stability of the CGO-PBC2-xFxO membranes under the CO2-containing atmosphere should be further studied. Fig. 3(a) presents the XRD patterns of the CGO-PBC0.5F1.5O membrane after exposing to CO2 with different concentrations at 900 ° C for 1 h. The membrane keeps its original structure and no carbonates phases appear with increasing CO2 content even to pure CO2, revealing an excellent stability under CO2 atmosphere at high temperature. Meanwhile, the membrane completely keeps its own structure without carbonate phases in the temperature range of 300-900 ° C under pure CO2 gas, as shown Fig. 3(b). From the XRD patterns, no obvious barium carbonate phase change was observed until the membrane treated in pure CO2 atmosphere for 20 h at 900 ° C, as shown Fig. 3(c). Fig. 3(d) shows the XRD patterns of all the CGO-PBC2-xFxO membranes after being treated in CO2 atmosphere for 20 h. The stability of crystal structure is gradually strengthened with increasing Fe-doping amount from x = 0 to 2, indicating that the donor substitution Fe for Co may increase the membranes stability against CO2.
Fig. 4 shows the oxygen permeation fluxes through the CGO-PBC2-xFxO membranes using He and CO2 as sweep gases at 925 ° C. The oxygen permeation experimental data of CGO-PBF2O membrane was not obtained due to its poor mechanical strength. CGO-PBC0.5F1.5O, containing the highest Fe content but the lowest Co content with the composition range covered, exhibits the highest oxygen permeation flux at CO2-containing atmosphere. The highest values of 2.71 × 10-7 and 2.46 × 10-7 mol cm-2 s-1 are achieved under 50 mol% He/CO2 and pure CO2 atmosphere at 925 ° C, respectively. As expected, the oxygen permeation fluxes decrease with increasing Co content in the CGO-PBC2-xFxO membranes, due to decomposition of the PBC2-xFxO phase. However, the oxygen permeation flux of CGO-PBC0.5F1.5O is much higher than those of the other membranes, and the actual mechanism is not clear. Only a slight decrease of the oxygen permeation flux through all the samples was observed when the sweep gas changed from 50 mol% He/CO2 to pure CO2. This behavior is different from the previous reports on the perovskite membranes[30]. For the BaCo0.4Fe0.4Nb0.2O3-δ and Ba0.5Sr0.5Co0.8Fe0.2O3-δ membranes, the oxygen permeation fluxes dropped to near zero immediately upon exposure of the membranes to a CO2-rich sweep gas[31] and [32]. Compared to the perovskite-type membranes, the CGO-PBC2-xFxO membranes exhibited good CO2-tolerance.
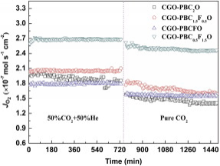 | Fig. 4 Time dependence of oxygen permeation flux through CGO-PBC2-xFxO membranes with different sweep gases; Condition: thickness, 1 mm; T, 925 ° C; air, 300 ml |
Fig. 5 (a) and (b) presents the effect of the flow rate of pure CO2 as the sweep gas on the oxygen permeation flux through the CGO-PBC2-xFxO membranes at different air flow rates. As displayed, the oxygen permeation fluxes increase with increasing CO2 flow rate, because the higher CO2 flow rate would dilute the permeated oxygen concentration and lower the oxygen partial pressure on the sweep side. For instance, when the CO2 flow rate changes from 20 to 100 ml min-1, the oxygen permeation fluxes of CGO-PBC0.5F1.5O membranes increase drastically from 2.28 × 10-7 to 2.71 × 10-7 mol cm-2 s-1 at 925 ° C, as shown in Fig. 5(b). A further increase of the air flow rate from 150 to 300 ml min-1 does not lead to a further obvious increase of the oxygen fluxes as compared Fig. 5(a) with Fig. 5(b). The reason why the effect of the change of air flow rate on the oxygen flux was negligible at a large air flow rate was that the change in the air flow rate did not cause apparent changes of the oxygen partial pressure in the upstream. This result was in agreement with the result of Wei et al.[33].
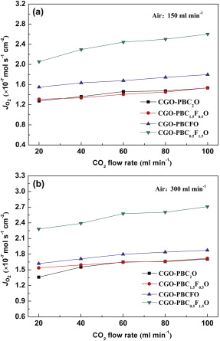 | Fig. 5 Effects of the gas flow rates on the oxygen permeation flux through the CGO-PBC2-xFxO membranes: (a) air flow, 150 ml min-1; (b) air flow, 300 ml min-1; Condition: thickness, 1 mm; T, 925 ° C. |
Oxygen permeation through the dual-phase membranes is controlled by two factors: one is diffusion rate of oxygen ions through the CGO phase and counter electrons through the PBC2-xFxO phases; the other is the surface oxygen exchange rate across the gas/solid interface. Sample CGO-PBC0.5F1.5O with excellent chemical stability was selected to investigate the thickness dependence of oxygen permeation, as illustrated in Fig. 6(a). The driving force for oxygen permeation is theoretically expressed by the following equation[34] and [35]:
equation(1)
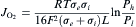

where F is the Faraday constant, R is the gas constant; T is the temperature; L is the thickness of the membrane; σ e and σ i stand for the electronic and oxygen ionic conductivity, respectively; P h and P l represent the oxygen partial pressure on the sweep side and feed side, respectively. When ln (P h/P l) is constant, the oxygen permeation flux (Jo2) should show liner function relation with the reciprocal of membranes thickness (1/L) over the whole temperature range if the rate-determining step of the oxygen permeation is bulk diffusion. Fig. 6(a) shows the permeation against the reciprocal of thickness (1/L) at 800-925 ° C. It can be seen obviously that the oxygen permeation flux increases proportionally with 1/L in the range of the membrane thickness of greater than 0.8 mm, revealing that the bulk diffusion is the rate-limiting step. When the membrane thickness is less than 0.8 mm, the flux increases a bit little and deviates from the linear trend, indicating that the reaction of the molecular oxygen with the membrane surface starts to be the limiting step. The maximum oxygen permeation flux is achieved as 4.20 × 10-7 mol cm-2 s-1 at 925 ° C with 0.6 mm thickness.
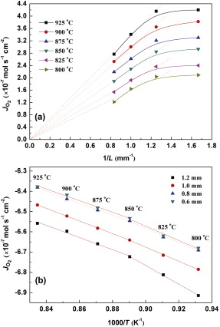 | Fig. 6 (a) Thickness dependence of oxygen permeation flux through CGO-PBC0.5F1.5O membrane; (b) related Arrhenius plots; Conditions: He, 100 ml min-1; air, 300 ml min-1. |
The activation energies for oxygen permeation of these CGO-PBC0.5F1.5O membranes with various thicknesses are shown in Fig. 6(b), which reveals that the membrane with 1.2 mm thickness possesses two activation energies for oxygen permeation in the temperature range 800-925 ° C. The reason why the slope changes at about 850 ° C may be the order-disorder transition of oxygen vacancies or the transition between bulk-diffusion limit and oxygen surface exchange limit according to the literature[36], [37] and [38].
Some research groups reported that some impurity phases were observed on both sides of dual-phase membranes after oxygen permeation measurements[39] and [40]. Fig. 7(a) and (b) shows the XRD patterns of the permeation and feed sides of the CGO-PBC2-xFxO membranes after oxygen permeation tests, respectively. In comparison with the fresh membranes, the composite of used membranes retains the major phases of CGO and PBC2-xFxO on the permeation sides, but serious damage appears on the feed sides. The impurity peaks on the feed sides are indexed primarily as some cobalt oxides, such as CoO, BaCoO3, BaPrO3 and other compounds, owning to the decomposition of PBC2-xFxO phases. With increasing Fe concentration in CGO-PBC2-xFxO, these impurity peaks become weak or even disappear. For example, both sides of CGO-PBC0.5F1.5O membrane can keep its integrated structure without impurity phases, revealing that the chemical stability of CGO-PBC2-xFxO membranes is improved with decreasing x level. SEM observation in combination with EDX analysis shows the microstructure and phase composition of the CGO-PBC2O membrane after oxygen permeation test.
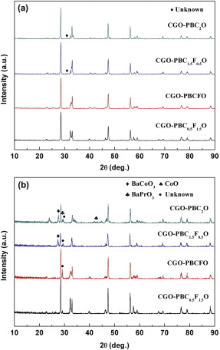 | Fig. 7 XRD patterns of CGO-PBC2-xFxO membranes after oxygen permeation experiment: (a) permeation side; (b) feed side. |
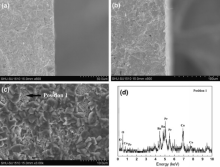
Fig. 8(a) and (b) shows the microstructure of the CGO-PBC2O cross-section of permeation and feed sides, which remains dense and almost unchanged, revealing a good stability of the composite membrane as compared with the perovskite membranes. Some porous grains are observed by SEM on the feed side surface of the used membrane, as shown in Fig. 8(c). The EDS analysis (Fig. 8(d)) confirms that the grains are close to cobalt oxides (e.g., a composition of 35.31 at.% O, 38.59 at.% Co, 10.52 at.% Ba, 15.58 at.% Pr), which is well consistent with XRD results. These impurities appeared on the feed side due to high oxygen partial pressure gradient[41].
The CGO phase and PBC2-xFxO phase show good chemical compatibility during the operation. With increasing Fe content in CGO-PBC2-xFxO, the oxygen permeation and structure stability are improved in CO2-rich atmosphere. The oxygen permeation flux can be enhanced by increasing the CO2 flow rate, whereas it hardly increases with increasing air flow rate at a large value. The highest oxygen flux in the pure CO2 atmosphere is achieved at the higher limit of Fe in the CGO-PBC2-xFxO, x = 0.5, where it is 2.71 × 10-7 mol cm-2 s-1 for the 1 mm thickness at 925 ° C. The oxygen permeating process is found to be controlled by bulk diffusion for the CGO-PBC0.5F1.5O membrane with thickness more than 0.8 mm, while it is mainly influenced by the surface oxygen exchange rate of membrane with thickness less than 0.8 mm. The variation of the mechanism of the activation energy in oxygen permeation is not clear. Compared with the other samples, CGO-PBC0.5F1.5O membrane possesses a high oxygen permeation flux and an excellent structural stability under pure CO2 atmosphere, revealing that the dual-phase membrane CGO-PBC0.5F1.5O is a great potential candidate material for separating oxygen from air in the oxy-fuel combustion.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|