Highly mesoporous ZnO and γ-Al2O3 nanowires (NWs) are both synthesized by a hydrothermal method using commercially available porous anodic aluminium oxide (AAO) as template. AAO membrane acts as template for ZnO NWs and both as template and precursor for γ-Al2O3 NWs. The formation of intermediate phases of porous Zn6Al2(OH)16CO3 and boehmite (γ-AlOOH) were observed, both occurring during the hydrothermal synthesis of porous ZnO and γ-Al2O3 NWs, respectively, and disappearing after annealing at 600 °C. This novel template-assisted hydrothermal process leads to the formation of porous ZnO and γ-Al2O3 NWs (specific surface area of 192 m2 g-1 and 263 m2 g-1, respectively), showing pore sizes around 4 nm in diameter. The influence of the reaction parameters on the nanostructure morphology was also investigated. A ZnO seed layer, deposited on the AAO channels prior to the hydrothermal synthesis, leads to more compact ZnO nanowires (99 m2 g-1) protecting the AAO host from the chemical attack of the precursor solution.
The preparation of nanomaterials with a good control and reproducibility of the process plays an important role in nanoscience and nanotechnology[1], [2], [3], [4] and [5]. Indeed, without control on the whole process, the development of advanced devices is limited. In addition, the use of simple technologies and earth abundant elements are considered valuable for reaching the goal of reproducing these processes at an industrial level. For instance, zinc oxide was proved to be quite effective in dye-sensitized solar cells, water splitting, energy nanogeneration and even in biomedical devices[6], [7], [8], [9] and [10]. In particular, the synthesis of ZnO nanowire (NW) arrays for energy harvesting applications could benefit from the presence of a supporting host membrane, allowing the growth of micrometer-long and vertically oriented NWs, separated one from each other by an insulating and well mechanically supported matrix.
The hydrothermal method is a conventional technique used to synthesize nanostructured and crystalline ZnO on flat substrates, and self-standing nanowires can be grown[6], [11], [12] and [13]. The synthesis of porous and mesoporous ZnO nanostructures was also reported by several methods, e.g. electrodeposition, surfactant-assisted growth[14] or thermal oxidation[15], obtaining mesoporous films[16] and [17], hollow and mesoporous microparticles[18], porous nanobelts[19] and even mesoporous nanowires[20]. Due to the noticeable surface area, these porous structures were proposed for different applications, such as photocatalyst in the degradation of dyes[16], photoanodes for a CdS quantum dot-sensitized solar cell (QSSC)[21], and gas sensor for volatile organic compounds (VOCs)[22].
Up to date, the hydrothermal approach was rarely combined with a template-assisted method, which gives the advantage of synthesizing ZnO nanowires well supported and separated from each other by means of an insulating membrane[23], [24] and [25]. One of the mostly used templates for supporting both inorganic[26] and [27] and organic[28] and [29] structures is the porous anodic aluminium oxide (AAO), prepared from anodic oxidation of aluminium foils or films[30] and [31]. Some authors reported on the synthesis of mesoporous ZnO nanowires confined into mesoporous silica[32] or the channels of a porous alumina membrane by either surfactant - assisted electrodeposition method[33] or co-precipitation from a precursor solution[34]. Despite the good quality of the synthesized material, they obtained a few μ m long nanowires and did not measured the pore volume and specific surface area of the obtained materials. In another work, Feng et al.[35] obtained the deposition of ZnO material inside the AAO channels, using a paired-cell deposition from a ZnO precursor solution, however, resulting in highly rough, polycrystalline and sometimes discontinuous material.
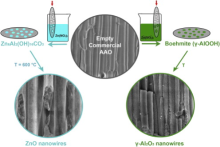 | Fig. 1 Scheme of the two synthetic routes to obtain mesoporous ZnO nanowires (left panel) and g-Al2O3 nanowires (right panel). |
In the present work, we report on the hydrothermal synthesis of highly mesoporous ZnO nanowires confined into the channels of commercially available AAO membranes (see Fig. 1, left panel). The proposed 1D ZnO-templated nanostructures can be customized depending on the host membrane features, i.e. channel size and length, and are easy to handle. In addition, since they are supported in an insulating membrane and separated one from each other, these mesoporous ZnO nanowires can be easily electrically connected with electrodes from the top and the bottom of the supporting membrane[28] without compromising mechanical compactness and stability. Therefore, they can easily be integrated into a working device for photocatalytic, energy photoconversion or gas sensing purposes, which would benefit from the high surface area of the mesoporous structure.
Moreover, here we show the preparation of vertically oriented and mesoporous γ -Al2O3 NWs, obtained by a self-templating process of the initially amorphous alumina membrane when immersed in a precursor solution. In particular, we first obtained oriented and porous boehmite 1D nanostructures and, after a thermal treatment, γ -Al2O3 NWs (Fig. 1, right panel).
Boehmite (γ -AlOOH) and its dehydrated alumina form (γ -Al2O3) are materials with high surface area that mainly serve as both catalyst and catalyst support, and are extensively used for the preparation of various optical and electronic devices[36], [37], [38] and [39]. However, it is difficult to synthesise these materials with good crystallinity in aqueous solutions because of the very fast hydrolysis rate of the alumina precursors, and therefore there is a limited number of reports on alumina nanostructures with well-resolved morphologies[40], [41], [42] and [43]. In contrast, 1-dimensional alumina nanobelt and nanowires were obtained by electrochemical and thermal assisted approaches[44] and [45].
Here we report the fast and one-pot synthesis of mesoporous γ -Al2O3 nanowires starting from an amorphous anodic alumina membrane. The use of a material that drives and templates its own nanostructuration has the advantage of ensuring a high reproducibility of the synthesized nanostructures. In addition the further removal of the template is no longer necessary. The high surface area of both the boehmite nanostructure and the γ -Al2O3 nanowires proposed in this work allows their use as good catalysts and catalyst supports.
In a typical experiment, ZnO nanostructures were synthesised by a hydrothermal method within the channels of commercial AAO with nominal pore size of 200 nm and thickness of 60 μ m (Anodisc® from Whatman, 47 mm in diameter), as previously reported[46].
The AAO membranes were first calcined at 600 ° C for 2 h at a heating rate of 1 ° C min-1. Afterwards they were immersed in a ZnO growth solution consisting of 50 mmol/L zinc nitrate hexahydrate (Zn(NO3)2· 6H2O), 25 mmol/L hexamethylenetetramine (HMT), 1.5 mmol/L polyethyleneimine (PEI, MW = 800 g mol-1, end-capped) and 320 mmol/L ammonium hydroxide in bi-distilled water and kept reacting at 88 ° C under stirring for a time period ranging from 0.5 to 16 h (samples AAO-ZnO). A further annealing step was performed on some selected samples at 600 ° C for 2 h at a heating rate of 1 ° C min-1 (sample AAO-ZnO_c).
A variation from the typical experiment consisted of immersing the AAO membrane in a ZnO seed layer (SL) solution prior to the ZnO nanostructures growth. In details, the AAO membrane was impregnated with a solution of 10 mmol/L zinc acetate in ethanol under vacuum conditions. After the impregnation, the membrane was dried in air. The impregnation was repeated 3 times. After the third impregnation, the AAO membrane was heated up until 350 ° C for 20 min (heating rate 5 ° C min-1). The further ZnO growth was performed in standard way as explained above (sample AAO-ZnO-SL and, after annealing at 600 ° C, sample AAO-ZnO-SL_c).
The reference sample (sample AAO-AlOOH, being constituted by 1D nanostructures of γ -AlOOH) and γ -Al2O3 NWs (sample AAO-Al2O3) were synthesized by a hydrothermal method in a similar way to the ZnO nanostructures, but without the ZnO precursor. A disc of AAO membrane was immersed in a growth solution containing 25 mmol/L hexamethylenetetramine (HMT), 1.5 mmol/L polyethyleneimine (PEI, MW = 800 g mol-1, end-capped) and 320 mmol/L ammonium hydroxide in bi-distilled water and kept reacting at 88 ° C for 2 h under stirring (sample AAO-AlOOH). In order to obtain the γ -Al2O3 nanostructures, the growth was followed by annealing at 600 ° C for 2 h with a heating rate of 1 ° C min-1 (sample AAO-Al2O3).
The morphology of the ZnO-impregnated samples was characterised by field emission scanning electron microscopy (FESEM, ZEISS Dual Beam Auriga). Nitrogen sorption isotherms were performed with Quadrasorb SI instrument (Quantachrome) and graphs are reported in adsorbed volume of gas at Standard Temperature and Pressure conditions (STP). Multipoint BET surface area was measured within the relative pressure range of p/p0 = 0.1-0.3. Non-Local Density Functional Theory (NLDFT) equilibrium model was also used to estimate the pore volume and the pore size distribution of the samples. X-ray diffraction (XRD) system with Cu-Kα X-ray tube (λ = 0.1542 nm) and an accelerating voltage of 40 kV was used to characterize the crystalline structures of both ZnO confined into the pores of the AAO and the AAO host itself. To optimize the signal to noise ratio of the spectra, long acquisition time per each step was used (15 s per step, with step size 0.02° ).
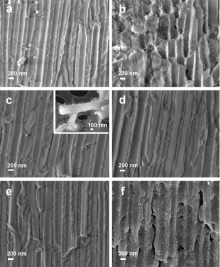
The cross-section FESEM images of the AAO membranes exposed to ZnO growth solution for different periods of time (from 0.5 to 16 h) are presented in Fig. 2. This figure clearly shows the nucleation of the ZnO within the channels of the alumina membrane. The 0.5 h reaction (Fig. 2(a)) results in almost empty and unmodified AAO channels. Only some isolated ZnO nanostructures are observed along the channels. After 1 h of reaction (Fig. 2(b)) fibre-like structures can be distinguished inside the template channels. This low-density fibre network represents the initial step of formation of the confined mesoporous ZnO crystals. After only 2 h (Fig. 2(c)) the ZnO nanostructures completely filled the 60 μ m long channels of the alumina membrane. The inset in Fig. 2(c) shows a single nanowire after the AAO template dissolution, confirming that the ZnO nanowires are a replica of the AAO template channels. However, a larger nanowire diameter (about 300 nm) is observed with respect to the AAO template channel (200 nm). The internal structure of the ZnO nanowires is highly porous, due to the growth mechanism consisting in the accretion of fibre-like crystallites. In addition, the NW array did not show a smooth surface but a highly irregular and porous one derived from the surface morphology of the alumina channels. This is caused by a side reaction taking place during the ZnO growth step, where the alumina is subjected to a corrosion process that will be broadly discussed below. This corrosion process modifies the surface of the alumina channels and, as a consequence, the external surface of the ZnO NWs shows some irregularities.
urthermore, longer reaction times (i.e. for 3, 5 and 16 h) result in a progressive damage of the alumina template (from Fig. 2(d) to (f)). After 3 h and 5 h of reaction the corrosion of the alumina walls increases, as evidenced by the higher porosity of the walls with respect to the original AAO material (which can be considered almost analogous to the 0.5 h-reacting AAO membrane, shown in Fig. 2(a)). The extreme case of the alumina wall destruction is observed in Fig. 2(f), depicting the AAO-ZnO sample after 16 h reaction. The characteristic anodic alumina form is no longer preserved: cracks, highly rough channel surfaces and porous cavities are visible throughout the whole membrane. Also the already formed ZnO nanowires are heavily damaged, and probably the ZnO nanostructures started to dissolve at a certain time until completing 16 h reaction. In particular, the lack of Zn2+ ions, because of the depletion of the zinc precursor in the growth solution after a prolonged reaction time, leads to porous ZnO nanostructures[6]. Additionally, the still high concentration of OH- ions generates an increase of the solution pH and Ostwald ripening process, leading to a profound erosion of both the AAO template and the ZnO nanostructures[6]. A side-effect of this erosion process is the increase of fragility of the final AAO-ZnO samples.
It is worth to mention that in all the synthesized samples, the template corrosion results in nanowires larger in diameter than the original AAO channels, similarly to the AAO-ZnO sample after 2 h growth[46]. This observation further confirms the increasing level of corrosion of the alumina membrane.
Besides, with this time-dependent growth study, it was established that 2 h is an optimal reaction time for obtaining from the one hand porous ZnO NWs with a smooth and slightly compact surface and, from the other hand a still robust porous alumina membrane, which can be still easily handled. A complete filling of the alumina channels was achieved and wurtzitic ZnO nanostructures were formed.
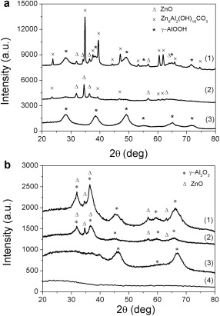
The XRD pattern of the AAO-ZnO sample after 2 h of growth reaction (curve 1, Fig. 3(a)) evidences the presence of several crystalline peaks, attributed to the formation of a mixed phase containing both Zn and Al compounds. First, the small and sharp peaks at 32.0° , 34.1° , and 36.4° are the (100), (002), and (010) reflections typical of the wurtzite crystalline structure of ZnO. Second, the intense and sharp peaks at 23.7° , 39.5° , 47.0° , 60.5° , and 61.8° correspond to the (006), (015), (018), (110), and (113) reflections of a mixed Al and Zn phase, respectively, i.e. zinc aluminium carbonate hydroxide (Zn6Al2(OH)16CO3)[47]. Furthermore, the presence of boehmite (γ -AlOOH) is confirmed by the broad peaks at 28.0° , 39.5° , and 49.0° corresponding to the (120), (031), and (051) reflections, respectively (JCPDS No. 21-1307).
The annealing step at 600 ° C for 2 h (sample AAO-ZnO_c) led to the decomposition of the (CO3)2- units in the form of CO2, allowing the further conversion of the Zn present in the interediate phase Zn6Al2(OH)16CO3 to wurtzitic ZnO and to γ -Al2O3, as confirmed by the XRD pattern of curve 1 in Fig. 3(b). The broader peaks at 32.0° , and 36.4° are attributed to the superposition of both these wurtzitic ZnO peaks with the (220) and (311) reflections of the γ -Al2O3, respectively, deriving from both the thermal conversion of the boehmite and zinc aluminium carbonate hydroxide intermediate products. In particular, the boehmite reflections at 28.0° , 39.5° , and 49.0° are no longer visible in the spectrum 1 of Fig. 3(b), whereas new broad peaks at 46.8° , 59.2° and 67.2° corresponding to the (400), (511), and (440) reflections of γ -Al2O3, are noticed (JCPDS No. 10-042). We attribute the origin of the γ -Al2O3 to the crystalline walls of the templating alumina.
For a better understanding of the chemical process that the AAO template underwent during the ZnO impregnation and thus to explain the FESEM and XRD results, a reference sample was prepared (see the Experimental section). An AAO membrane was immersed in the growth solution for 2 h without the addition of the zinc precursor (Zn(NO3)2· 6H2O) to the solution. The alumina morphology, shown in Fig. 4(a), changed with respect to the starting empty template (assumed analogous to Fig. 2(a)). The smooth walls of the starting membrane became rough with needle-like protrusions, making difficult to determine which part corresponds to the walls and which one to the channels. Despite the starting AAO template is an amorphous material, the XRD pattern after the reaction (curve 3, Fig. 3(a)) shows the typical diffraction peaks attributed to boehmite (γ -AlOOH). In fact, also the needle-like morphology observed in the chemically treated AAO template (Fig. 4(a)) is typical of boehmite[40] and [48]. The sample is thereafter called AAO-AlOOH, since templated 1D nanostructures of γ -AlOOH were obtained. The transition of bulk Al2O3 from amorphous material to boehmite was previously reported[49] and [50] by means of the alumina reaction with HMT or NH4OH. In the present work, the use of both ammonium hydroxide and hexamethylenetetramine induced the formation of boehmite through a hydration reaction of the Al2+ ions of the porous alumina membrane. After annealing at 600 ° C for 2 h, the boehmite crystal structure is converted to crystalline γ -Al2O3, as shown in the XRD pattern of curve 3 in Fig. 3(b). Therefore, similarly to the AAO-ZnO sample after 2 h reaction and thermal treatment, the boehmite reflections are no longer present in this spectrum. The (400), (511) and (440) reflections of γ -Al2O3 are clearly observed. The (220) and (311) reflections are also slightly observed at 32.0° and 36.4° , as also previously reported[29].
lt is worth to note that the calcination of the starting amorpho s AAO membrane does not promote any crystallization process, as confirmed by the XRD pattern displayed in curve 4 of Fig. 3(b). It can therefore be assessed that the AAO amorphous template changed its anodized porous form into porous 1D nanostructures of boehmite (Fig. 4(a), sample AAO-AlOOH) during the reaction step of 2 h and further into mesoporous γ -Al2O3 nanowire array (Fig. 4(b), sample AAO-Al2O3) after further thermal treatment at 600 ° C. The growth of crystalline alumina nanowires was previously reported starting from aluminium precursors[51] or from AAO membranes immersed in NaOH solutions[52], where under specific conditions of concentration and time the membrane itself formed nanotubes instead of getting dissolved.
This section explains how the addition of a ZnO seed layer to the starting AAO membrane can modify some characteristics of the AAO-ZnO samples, i.e. surface area and preferential growth of phases, as well as leading to a reduced formation of boehmite and further of γ -alumina.The seed layer is normally applied in the synthesis of ZnO nanowires on flat substrates with the scope of forming a thin film of crystalline ZnO acting as nucleation sites for the further ZnO NW formation[6], [12] and [13]. This thin layer (< 20 nm) also facilitates a good adhesion of the NWs to the matrix. In our case, the impregnation of the AAO membrane into the seed layer solution leads to a coverage of the alumina walls by the crystalline seed layer. This thin layer delays the contact between the template and the growth solution, thus protecting the alumina membrane from the chemical attack. Fig. 5(b) shows the FESEM image of the NWs prepared after depositing the seed layer, and then growing the ZnO for 2 h and annealing the AAO-ZnO sample at 600 ° C (sample named AAO-ZnO-SL_c). To better compare the seed layer effect in the final material, Fig. 5(a) also shows the annealed sample AAO-ZnO_c, i.e. without seed layer, after the same growth time, i.e. 2 h. The samples without (Fig. 5(a)) and with seed layer (Fig. 5(b)) look similar at low magnification: both NWs have a smooth surface and the alumina walls appear analogous. In both cases, part of the alumina was damaged by the growth solution and therefore the AAO walls show a porous surface. However, at higher magnifications (see the insets of Fig. 5), the structure of the nanowires present several differences. In both cases the NWs are highly porous, however the AAO-ZnO_c NWs are composed by agglomerates of small nanostructures. In contrast, the AAO-ZnO-SL_c NWs have a compact external shell and a lamellar-like nanostructured core. It can be therefore confirmed that the external and compact shell of the nanowires is related to the presence of the seed layer, inducing the formation of a compact layer of ZnO at the first stages of the ZnO growth. Furthermore, damage of the alumina template and depletion of the reactant interrupt this compactness, resulting in a mesoporous nanostructured core.
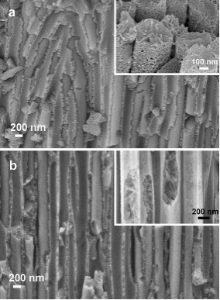 | Fig. 5 FESEM image of (a) AAO-ZnO_c and (b) AAO-ZnO-SL_c samples. All samples were exposed to the growth reaction conditions for 2 h and annealed at 600 ° C for 2 h. |
The XRD patterns of the AAO-ZnO-SL sample before and after annealing (curve 2, in Fig. 2 and Fig. 3, respectively) show the typical (100), (002) and (101) reflections of wurtzitic ZnO structure at 31.8° , 34.4° , and 36.2° , respectively. Very slight and broad peaks of boehmite are also observed in the sample AAO-ZnO-SL before annealing corresponding to the (120), (031), (051), and (200) reflections at 28.1° , 38.3° , 48.9° , and 49.2° , respectively. This implies a strong predominance of wurtzitic ZnO NWs into the porous alumina matrix and a very weak formation of the boehmite phase thanks to the protective role of the ZnO seed layer with respect to the alumina surface.
In the AAO-ZnO-SL_c sample (curve 2, Fig. 3(b)) the boehmite reflections are no longer visible, whereas slight and broad bumps attributed to the (400), (511) and (440) reflections of γ -Al2O3 are observed. As also observed for the sample AAO-ZnO_c in curve 1, here also a broadening of the wurtzite reflections at 32.0° and 36.4° are attributed to the superposition of both these wurtzitic ZnO peaks with the (220) and (311) reflections of the γ -Al2O3, respectively. As reported above, the presence of γ -Al2O3 is attributed to the crystalline walls of the templating alumina.
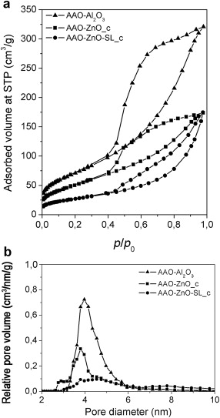
The N2 sorption isotherms of the samples AAO-Al2O3, AAO-ZnO_c and AAO-ZnO-SL_c are reported in Fig. 6(a) together with the isotherm of the sample AAO-Al2O3 for ease comparison. In all the three cases, the hysteresis in the pressure ranges p/p0 = 0.4-0.9 indicate a high level of textural porosities of the samples. The sample AAO-Al2O3 with γ -Al2O3 nanowires shows the highest surface area, equal to 263 m2 g-1. This value confirms the highly porous structure of the γ -Al2O3 nanowires, as discussed above. The reduction of the surface area of the ZnO-impregnated samples, equal to 198 m2 g-1 for AAO-ZnO_c and 99 m2 g-1 for AAO-ZnO-SL_c, confirms the presence of ZnO within the alumina channels constituted by γ -Al2O3 material. Additionally, the seed layered sample shows the lowest surface area, confirming the lower level of damage of the AAO membrane carried out by the growth solution. Similarly, the DFT (density functional theory) pore size distribution ( Fig. 6(b)) shows a consistent reduction of the relative pore volume, from 0.75 cm3/nm/g (for AAO-Al2O3) to 0.35 and 0.1 cm3/nm/g for the samples AAO-ZnO_c and AAO-ZnO-SL_c, respectively. We note that the pore size distribution of both AAO-Al2O3 and AAO-ZnO_c is quite uniform and centred at about 4.0 nm and 3.8 nm, respectively, and can thus be denoted as unordered mesoporosities. The sample AAO-ZnO-SL_c shows a large broadening in the pore size distribution, attributed to the lamellar-like morphology of the ZnO NWs core.
Mesoporous ZnO and γ -Al2O3 NWs were successfully synthesized within an amorphous AAO host by using a novel template-assisted hydrothermal approach. The study of different ZnO growth times during the alumina immersion in the Zn-precursor solution demonstrated that hydrothermal reaction for 2 h yields to mesoporous ZnO nanowires, completely filling the host membrane, thus avoiding serious damages to the whole sample. Together with wurtzite-like nanostructures, a mixed phase between Al and Zn (i.e. Zn6Al2(OH)16CO3) and a boehmite crystalline structure (constituting the alumina walls) were also observed for the as-prepared samples. However these intermediate phases can be further converted into wurtzitic crystalline ZnO and γ -Al2O3 after the annealing process, without losing the porosity features. In a separate process, the initially amorphous commercially available AAO membrane immersed in the hydrothermal solution without the zinc precursor changed its chemical and morphological structure to needle-like crystalline boehmite 1D nanostructures and, after further annealing process, to highly porous γ -Al2O3 NWs with mesopores of about 4 nm and a remarkable surface area of 263 m2 g-1. In order to prevent the chemical attack of the alumina host during the ZnO growth, the deposition of a ZnO seed layer was carried out prior to the ZnO growth reaction. In this way smoother and more compact ZnO nanowires, showing a dense external shell and a lamellar-like nanostructured core, were obtained.
These templated and highly porous 1D nanostructured materials, being easy to synthesize, oriented and well supported in a host matrix, can find potential application in different fields. ZnO nanowires, having a very high aspect ratio and surface area, can be for example exploited as gas sensor or photoanodes in dye-sensitized solar cells (DSSCs), as well as mechanical energy nano-harvesters, being separated one from each other by an insulating matrix, and well mechanically supported. In addition the γ -Al2O3 nanowires, with their high surface area and sharp pore size distribution, can be used as highly porous and oriented catalysts and catalyst supports.
The help of Dr. Angelica Chiodoni for FESEM imaging is gratefully acknowledged.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|