Rubbers have been well accepted for modifying brittle epoxies but rubber modified epoxies usually posses lowered tensile strength though enhanced ductility and fracture resistance. In this work, a polyethylene glycol (PEG-4000) is used to modify diglycidyl ether of bisphenol A/methyltetrahydrophthalic anhydride system for enhancing cryogenic tensile strength, ductility and impact resistance. The results display that the cryogenic tensile strength, ductility (failure strain) and fracture resistance (impact strength) are all enhanced for the modified epoxy system at proper PEG contents. The maximum tensile strength (127.8 MPa) at the cryogenic temperature (77 K) with an improvement of 30.1% is observed for the modified system with the 15 wt% PEG content. The ductility and impact resistance at both room temperature and cryogenic temperature are all improved for the modified epoxy system with proper PEG-4000 contents. These observations are explained by the positron annihilation lifetime spectroscopy results and scanning electron microscopy results. Moreover, the glass transition temperature decreases slightly with increasing PEG content.
Due to their easy fabrication, good electric insulating and mechanical properties etc., thermosetting epoxy resins have been well accepted as matrices for fiber reinforced composites applied in cryogenic engineering fields in the temperature range of liquid helium (4.2 K), liquid hydrogen (20 K), liquid nitrogen (77 K), liquid oxygen (90 K), and liquid methane (112 K) [1], [2], [3], [4], [5], [6] and [7]. However, as a kind of thermosetting resins, the intercrossed molecular chains make epoxy resins rigid and brittle, thus micro-cracking and even fracture of epoxy resins might happen when their thermal-stress induced stress intensity factor exceeded their fracture toughness as temperature decreased down from room temperature to cryogenic temperature [3] and [8]. This would result in the permeation of medium molecules such as helium, hydrogen, nitrogen, oxygen or methane molecules along the cracking passages when epoxy resins were used as matrices for composites [9]. Therefore, it is necessary to improve the cryogenic mechanical performance of epoxy resins to meet the high requirements by cryogenic engineering applications.
Blending of polymers with rubbers is an important area of research activity [10]. Rubbers are often used to modify brittle epoxy resins. Epoxy resins aimed for cryogenic engineering applications are also modified by using rubbers. Ueki et al. [3] designed several epoxy systems with different chemical structures and employed carboxyl-terminated butadiene–acrylonitrile rubber copolymer to modify epoxy resins. Nobelen et al. [7] used two carboxyl-terminated butadiene–acrylonitrile rubbers as modifiers to improve the cryogenic mechanical performance and investigated their cryogenic micro-cracking behavior. However, rubber modified epoxies showed enhanced ductility and fracture resistance such as impact strength but generally exhibited lowered tensile strength.
In this work, polyethylene glycol (PEG) with the molecular weight of 4000 g/mol (PEG-4000) is employed to modify the epoxy system of diglycidyl ether of bisphenol A (DGEBA)/methyltetrahydrophthalic anhydride (MeTHPA) to enhance their cryogenic tensile strength, ductility and impact resistance. First, the hydroxyls at the ends of PEG-4000 molecules would react with the curing agent (MeTHPA) and hence the PEG-4000 molecules would enter and control the network structure of the cured epoxy system. Second, the flexible PEG-4000 polymer chains, which are composed of ether bonds, are expected to improve the impact resistance at cryogenic temperature of cured epoxy resins. However, to our best knowledge, no work has been done so far on the cryogenic mechanical properties of epoxy resins modified by PEG-4000. Therefore, in this work, the cryogenic mechanical behaviors at 77 K of MeTHPA/DGEBA system modified by PEG-4000 are reported in terms of their cryogenic tensile and impact properties. The liquid nitrogen was used to create a cryogenic temperature (77 K) environment due to its less expensiveness compared to other cryogenic media. The mechanical properties at room temperature (RT) were also investigated for comparison.
The epoxy resin, DGEBA (CYD-128) with an epoxide equivalent of 184–194, was provided by Yueyang Refinery Plant, China. The curing agent, methyltetrahydrophthalic anhydride (MeTHPA), was bought from Shanghai Li Yi Sci. & Technol. Development Co. Ltd, China. Benzyl dimethylamine (BDMA) as the accelerator was obtained from Shijiazhuang Wells Electronic Material Co., Ltd, China. The PEG-4000 with the molecular weight of 4000 g/mol used in this work was purchased from Beijing Chemical Reagent Company. The chemical structure of PEG-4000 was shown in Fig. 1. The chemical structures of DGEBA and MeTHPA have been given in our previous paper [11].
60.0 g DGEBA was firstly mixed with 3.2, 6.7, 10.6 and 15.0 g PEG-4000, respectively and stirred at 60 °C for 30 min until a homogenous mixture was observed. The PEG-4000 content in the blended mixtures varied from 0, 5, 10, 15 and 20 wt%. 60 g MeTHPA and 1.5 g BDMA were added to the PEG-4000/epoxy mixtures at 60 °C, respectively, which were then degassed under vacuum for 30 min. Homogenous solutions were obtained, and casted in a preheated steel mold at 80 °C, then cured at 130 °C for 2 h, 150 °C for 10 h, and post-cured at 170 °C for 5 h. The dumbbell-shaped tensile specimens were prepared according to the ASTM D638-96. The impact specimens were prepared with the dimensions of 4 mm (thickness) × 10 mm (width) × 80 mm (length) according to the ASTM D-256.
The PALS characterization was conducted by an EG&GORTEC fast–slow coincidence system with a resolution of 180 ps at room temperature. The source of 22 Na (5 × 105 Bq) was sandwiched between two pieces of the samples and three million counts were collected for each spectrum. The positron lifetime ( τ3) and intensity ( I3) of the long-lived components were analyzed by the computer program “POSFIT88”.
The glass transition temperature ( Tg) of the cured specimens was measured via NETZSCH STA 409 PC at a heating rate of 10 °C/min under nitrogen atmosphere. The heating temperature ranges from 30 to 200 °C. The tensile properties of the cured samples were tested at RT and 77 K by an RGT-20A Reger mechanical tester, by using a 10 kN load cell with a crosshead speed of 2 mm/min. The clamps and the samples were dipped in a liquid nitrogen filled cryostat designed in our laboratory when the tensile samples were tested at 77 K.
The impact strength of the cured specimens was measured with a REGER impact tester. At least five specimens were tested for each composition. When the impact testing was performed at 77 K, the specimens were dipped in a liquid nitrogen filled cryostat for over 10 min, then the impact testing was quickly completed within a couple of seconds after taking the specimens out from the cryostat. During the impact testing, the temperature inside the samples would show no obvious change after the specimens were taken out from the cryostat for a few seconds and the temperature was approximately considered as 77 K [12]. The micrographs of the fracture surfaces of the impact specimens were taken by scanning electron microcopy (SEM, HITACHI S-4300). The fracture surfaces were cleaned with alcohol and spray coated with a thin layer of evaporated gold to improve their conductivity before the examination.
Positron annihilation lifetime spectroscopy (PALS) is an effective technique to evaluate the free volume of polymers and has been widely used in studying polymer systems at molecular level. The free volume is an important parameter for describing the structure of a polymer and has great influences on the mechanical properties of the polymer, mainly depending on the ability of the polymer segments to pack together by the rigidity of the backbone and the cross-link density. This technique utilizes the interactions between the positrons and the electrons from the host material to elucidate the free volume hole dimensions, which range from about 0.1 to 1 nm. The positronium lifetime ( τ3) has a strong correlation with the size of the free volume and is an indication of the average size of free volumes; the intensity ( I3) is an indication of the concentration of free volumes within the cured epoxy networks [7], [11], [13], [14] and [15].
The measured results by PALS for the positronium lifetime ( τ3) and the intensity ( I3) as a function of PEG-4000 content are exhibited in . It can be seen from that the τ3 value increases with increasing PEG-4000 content, which indicates that the average size of the free volumes within the PEG-4000 modified epoxy systems increases with increasing PEG-4000 content. And the I3 value for the modified epoxy systems is obviously larger than that for the unmodified epoxy system, which displays that the concentration of free volumes in the PEG-4000 modified epoxy networks is higher than that in the unmodified epoxy system. Fig. 2(a) and (b) shows the size of the simulated structures of single PEG-4000, DGEBA and MeTHPA molecule by using the well known Chemoffice3D Ultra 10.0 Software. It can be clearly seen that the flexible PEG-4000 chain with the molecule weight of 4000 g/mol used in this work is far larger than the small single epoxy molecule with the molecule weight of about 392.16 g/mol and the cure agent MeTHPA molecule with the molecule weight of 166.18 g/mol. The schematic illustration for the cured networks in the unmodified epoxy and the free volumes in the PEG-4000 modified epoxy systems is presented in Fig. 3. It shows that the distances between the networks around the PEG-4000 chains will increase when the PEG-4000 chains are introduced into the epoxy networks. Consequently, the size and concentration of the free volumes in the modified epoxy system will be increased by the introduction of PEG-4000.
| Table 1. Positronium lifetime τ3, intensity I3 and glass transition temperature ( Tg) of unmodified and PEG-4000 modified epoxy resins |
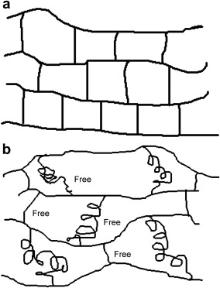 | Fig. 3. Schematic illustration of: (a) unmodified epoxy networks, (b) PEG-4000 modified epoxy networks. |
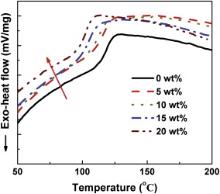
The thermal properties of the unmodified and PEG-4000 modified epoxy resins were measured by means of DSC technique. The DSC traces at a heating rate of 10 K/min are shown in Fig. 4 for the neat epoxy resin and PEG-4000 modified epoxy resins. It can be seen that there is only a single Tg for all of the compositions, which indicated the presence of miscibility [16]. The variation in Tg for the systems with respect to the PEG-4000 content is given in . It can be observed that Tg decreases with increasing PEG-4000 content. The chain flexibility, intermolecular attraction and cross-linking density etc., are the main factors influencing the glass transition temperature of polymers [17] and [18]. The introduction of flexible PEG-4000 polymer chains can make the polymer chains to move more easily and hence to decrease the Tg of the cured systems [19]. The PEG-4000 molecules with large free volumes increase the distances between the networks around the PEG-4000 chains and hence decrease their intermolecular attraction. The decreased intermolecular attraction can also make the polymer chains move easily and thus decrease the Tg of the PEG-4000 modified cured systems. Moreover, the free volume should be dependent on cross-linking density and cross-links may stop the molecular segments from packing together in an optimum manner [14]. So the increased size and concentration of the free volumes as exhibited by the PALS results corresponded to a decrease in the cross-linking density [14], which will lead to a decrease in the Tg of the cured systems [14] and [19]. Therefore, it is naturally observed that the Tg of the cured epoxy system decreases as the PEG-4000 content increases.
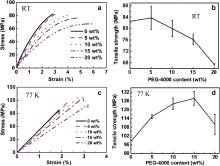
The stress–strain curves are shown in Fig. 5 for the unmodified and PEG-4000 modified epoxy resins at both RT and 77 K. From the two groups of stress–strain curves, the PEG-4000 modified epoxy resins exhibit more ductile behavior than the unmodified epoxy resins. Moreover, the epoxy resins show more brittle behaviors at 77 K than at RT. The data of the tensile strength, tensile modulus and failure strain of the epoxy resins at RT and 77 K were obtained from these stress–strain curves via the REGER testing software. The results are summarized in and also presented in Fig. 5.
| Table 2. Tensile properties at RT and 77 K of unmodified and modified epoxy resins |
It is shown in and Fig. 5 that the tensile strength at RT and 77 K shows completely different dependencies on the PEG-4000 content. The tensile strength at RT does not change apparently until the PEG-4000 content is increased to 10 wt% and then decreases with the further increase of the PEG-4000 content. However, the tensile strength at 77 K increases with increasing PEG-4000 content and reaches the maximum average value of 127.8 MPa for the modified system with the 15 wt% PEG-4000, showing an improvement of 30.1% over that (98.2 MPa) for the unmodified epoxy resin. Afterward, the tensile strength at 77 K decreases when the PEG-4000 content is further increased to 20 wt%, but is still higher than that for the unmodified epoxy resin.
The RT tensile strength is discussed first. It is well recognized that the internal residual stress in cured epoxy resins would be produced by bulk shrinkage occurring during the cooling process from the curing temperature to RT [20] and [21]. The internal stress within the cured bulk material can greatly affect the mechanical performance of the finished product by creating micro-cracks and voids. Conversely, the introduction of soft segments into epoxy resins can dramatically reduce this internal stress. Thus, the soft segments of ether bonds in flexible PEG-4000 polymer chains could reduce the internal stress within the chain networks of brittle epoxy resins and thus would contribute to the increase in the tensile strength at RT. On the other hand, as the PEG-4000 content is further increased beyond 10 wt%, the negative influencing factor due to introduction of too much flexible segments of PEG-4000 would reduce the tensile strength. Besides, the introduction of free volumes in PEG-4000 modified networks as indicated in (PALS result), Figs. 3 and 4 (DSC result) would lead to a decrease in the cross-linking density of the modified epoxy systems since free volumes would take space in the cured modified epoxy networks, and hence to a decrease in the tensile strength at RT [22]. Thus, when the PEG-4000 content is relatively low (≦10 wt%), the reduction of tensile strength caused by the above negative factors would be offset by the increment contributed by the above positive factor. As a result, the tensile strength at RT does not change apparently when the PEG-4000 content is increased up to 10 wt%. However, when the PEG-4000 content is further increased to 15 wt% or higher, the positive contribution may not be enough to offset the negative contribution to the strength. Consequently, the tensile strength at RT is reduced by the introduction of 15 and 20 wt% PEG-4000. The similar observation has been reported by other flexible modifiers [23], [24] and [25].
The cryogenic tensile strength is then discussed below. The tensile strength of epoxy resins increases with decreasing chain length of PEG-4000 because of shrinkage when cooling down to 77 K since the chain length affects the epoxy network structure density. Moreover, in the PEG-4000 modified epoxy systems, the plenty of soft flexible segments of ether bonds incorporated into epoxy networks would not be completely frozen and could still reduce the internal stresses via the bond motion. At 77 K, epoxy resins become more brittle and thus the reduction in internal stress will be more important to enhance the tensile strength at 77 K than at RT. The free volumes in the cured systems would be smaller at 77 K due to thermal shrinkage, which contributes to an increased intermolecular force compared to the RT case [11], also leading to a higher tensile strength at 77 K compared to the RT. Consequently, these would result in a higher tensile strength at 77 K compared to RT. On the other hand, as the content of PEG-4000 is further increased, the negative influencing factor due to introduction of too much flexible segments of PEG-4000 would dominate in determining the tensile strength. This is similar to the RT case and would not be discussed in detail for simplicity.
The tensile modulus of the epoxy resins at both RT and 77 K are also exhibited in . It can be seen that the tensile modulus at both RT and 77 K tends to decrease with increasing PEG-4000 content. The main reason can be attributed to the decrease in the rigidity of polymer chains and the cross-linking density caused by the introduction of flexible PEG-4000 chains in the PEG-4000 modified cured systems. Moreover, it can be observed that the tensile modulus at 77 K is larger than that at RT with the same composition. Similar results were reported in our other works [22] and [26]. This is because the binding force between atoms in the polymer chains and intermolecular force between the polymer chains at 77 K are greater than that at RT due to thermal shrinkage, which would make contribution to the increase of the tensile modulus.
The results for the failure strain of the unmodified and modified epoxy resins are also shown in . It displays that the failure strain at 77 K is lower than that at RT for all samples. This is because the free volumes become smaller and the mobility of macromolecules becomes lower at 77 K than at RT and moreover, the macromolecules are partially frozen up at 77 K, resulting in reduction of the ductility. also displays that the failure strain at both RT and 77 K increases consistently with increasing PEG-4000 content, showing the enhanced ductility by the addition of PEG-4000. This is attributed to the easier motilities by the introduction of flexible segments of ether bonds from PEG-4000 in the rigid epoxy network structure. The presence of free volumes due to the introduction of PEG-4000 provides room for facilitating polymer chain mobility while the cured epoxies are subjected to a tensile force, which also contributes to an enhancement in the failure strain.
The impact resistance is important for epoxy-based composites applied in aerospace and automotive industries etc [27] and [28]. Here the impact strength of cured epoxy systems is examined as a function of PEG-4000 content. Effects of the PEG-4000 content on the RT and cryogenic impact strength of the cured epoxy resins are shown in . The data for the impact strength are somewhat overlapped when considering the standard errors, thus the average values of the impact strength are used for discussion. It is clear from that the impact strength at both RT and 77 K of the PEG-4000 modified epoxy systems is higher than that of the unmodified epoxy resin. The impact strength at RT increases up to the maximum by adding the 15 wt% PEG-4000 and afterward decreases with the further increase of the PEG-4000 content. The maximum impact strength at 77 K occurs for the modified epoxy system with the 10 wt% PEG-4000 content. Moreover, the impact strength at 77 K does not change apparently when the PEG-4000 content is further increased from 10 to 20 wt%. The maximum improvements in the impact strength at RT and 77 K are 25.9% and 20.5% over that of the unmodified epoxy resin, respectively.
| Table 3. Impact strength at RT and 77 K of unmodified and PEG-4000 modified epoxy resins |
The RT impact strength is first discussed. Generally, the epoxy resins have highly cross-linked structures and are brittle because they are cured with the small molecular curing agents such as aliphatic or aromatic amides, anhydrides, etc. It is clear from Fig. 2 that both the DGEBA and MeTHPA molecules employed in this work are small. The cross-linking density of the cured unmodified epoxy system which was composed of small DGEBA and MeTHPA molecules is relatively high when compared to the large PEG-4000 modified epoxy systems as shown in Fig. 3. Thus, the low impact strength of the unmodified epoxy can be attributed to their relatively high cross-linking density [14], [19] and [24]. It is shown above that the size and concentration of free volumes increases as the PEG-4000 content increases (see ). The free volumes in the PEG-4000 modified epoxy systems can absorb energy by distorting themselves and providing motion room for the molecules in the network during impacting [7] and [29]. Moreover, the soft segments of ether bonds can reduce internal stress due to the stress relaxation by flexible ether segments in PEG-4000 and dissipate the impact energy by their segmental motion in molecular chains in the PEG-4000 modified epoxy systems when the samples suffer impact testing. Furthermore, the cross-linking density of the PEG-4000 modified epoxy systems is decreased by the introduction of PEG-4000 due to the increased average molecular weight of chain segments between cross-link points. This can also increase the impact strength. As a result, the impact strength at RT of the cured epoxy resins increases as the PEG-4000 content increases up to 15 wt%. As the PEG-4000 content is increased beyond 15 wt%, the impact strength is decreased. This is possibly because the cross-linking structure of the modified epoxy systems cannot keep their integrity in the microstructure due to introduction of too much amount of free volumes as exhibited in Fig. 3, leading to the decrease in the impact strength. This is similar to the previous reports [11] and [23].
At 77 K, the flexible groups (namely ether segments in PEG-4000) are not completely frozen. And the effect of the reduction in internal stress by flexible ether segments would be more important than that at RT and could dissipate the impact energy by their segmental motion in molecular chains [11] and [29], which contributes to the increase of cryogenic impact strength [25] and [29]. The free volumes within the cured epoxy system as indicated in and Fig. 3 become smaller but still exist and can also absorb much energy by distorting themselves and providing motion space for the molecules in the network during impacting at 77 K [7] and [29]. These positive factors together will make contribution to the increase of cryogenic impact strength of the PEG-4000 modified cured systems. As the PEG-4000 content is further increased beyond 10 wt%, the cryogenic impact strength of the cured systems does not change apparently. This is possibly due to the same reason as for the RT impact strength that the cross-linking structure of the modified epoxy systems may not keep their integrity in the microstructure due to introduction of too much amount of free volumes, leading to a decrease in the impact strength. The competing positive and negative factors would then lead to no apparent change in the impact strength.
The representative fracture surfaces of the unmodified epoxy resins and the PEG-4000 modified epoxy resins at RT and 77 K are presented in Fig. 6(a)–(h). From the SEM micrographs, no separate second phase can be found in the SEM images as shown in Fig. 6(c)–(h), which confirms the good compatibility between PEG-4000 and epoxy resin. In addition, the smooth and glassy surfaces as shown in Fig. 6(a) and (b) reveal the brittle failure of the unmodified epoxy resin. And the relatively rough and irregular appearance of the PEG-4000 modified epoxy resins with the 10 to 20 wt% of PEG-4000 are shown in Fig. 6(c)–(h), indicating the presence of plastic deformation before fracture. This is quite different from that for the unmodified epoxy resin. The difference between the unmodified epoxy and the modified epoxy systems originates from the energy absorbed during plastic deformation in the modified epoxy network, giving rise to the higher impact strength for the modified epoxy systems than for the unmodified resin.
The cryogenic tensile and impact properties of the DGEBA/MeTHPA system have been enhanced by the introduction of polyethylene glycol (PEG-4000). The results show that the addition of PEG-4000 at proper amounts has led to simultaneous improvements in the cryogenic tensile strength, ductility and impact resistance. The maximum tensile strength and the maximum impact strength at 77 K have been obtained for the modified epoxy system with proper PEG-4000 contents. The elongation at break at 77 K is also consistently increased by the addition of PEG-4000. These observations have been explained by the positron annihilation lifetime spectroscopy results and the SEM results. The glass transition temperature Tg is slightly decreased by the introduction of PEG-4000 and the decreasing degree depends on the amount of PEG-4000.
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (Nos. , and ).
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|
| 18. |
|
| 19. |
|
| 20. |
|
| 21. |
|
| 22. |
|
| 23. |
|
| 24. |
|
| 25. |
|
| 26. |
|
| 27. |
|
| 28. |
|
| 29. |
|