A uniform, compact, and well adherent conversion coating of magnesium hydroxide has been formed on bioresorbable magnesium disks by means of a hydrothermal technique. Electrochemical results indicate that the coating brings about a significant reduction in magnesium corrosion in phosphate buffered saline (PBS) solution. It is also observed that corrosion resistance of the coating increases with an increase in treatment time, which in turn, increases the coating thickness. The protective behavior of magnesium hydroxide coating is attributed to its chemical inertness in PBS solution. The coatings are found to be free from pores that reduce the direct contact between corroding media and underlying magnesium.
Magnesium (Mg) and its alloys have attracted considerable research interest for biomedical and automobile applications because of their promising properties such as biocompatibility, low density, high specific strength, castability, and appropriate hardness [1], [2], [3], [4], [5] and [6]. However, the high corrosion rate of Mg severely limits its usage in almost all of these applications [7] and [8]. Therefore, in order to utilize Mg in these applications, the corrosion rate of Mg needs to be slowed down [9] and [10]. An effective way to reduce the corrosion rate of Mg and its alloys is surface modification [7], [11] and [12]. Surface modification includes anodic oxidation [13], metal coating [14], chemical conversion coating [15], plasma electrolytic oxidation [16], and organic coating [17]. The surface modification by chemical conversion coating is widely used because of its easier operation, effectiveness, and lower cost [18]. Among the chemical conversion coatings, chromate conversion coating is one of the most effective and popular conversion coating to reduce the corrosion rate of Mg [19]. However, the use of chromate coating is being progressively restricted due to the high toxicity of the hexavalent chromium compounds; hence it is necessary to develop environment friendly replacements for chromate conversion coatings [11] and [20].
Different types of conversion coating such as phosphate [21], phosphate/permanganate [22], fluoride [23], stannate [24], phytic acid [25], rare earth element based conversion coatings [26] and [27] have been emerging as an alternative to chromate conversion coating to reduce the corrosion rate of the Mg and its alloys. Cui et al. [28] used neodymium-based conversion coating on Mg alloy as a substitute for toxic chromate conversion coating. They observed that post treatment of the phytic acid coating improves the corrosion resistance of the Mg alloy. Recently, we have also reported that post heat treatment of phytic acid conversion coated Mg shows improved corrosion resistance compared to non-heat treated phytic acid conversion coated Mg [29]. Cerium based conversion coatings have been used to decrease the corrosion rate of AZ31 magnesium alloy [30]. The effect of varying Ca2+ and4PO3- concentrations on the formation of calcium phosphate conversion coatings on Mg alloy was studied [31]. It was observed that low4PO3- concentration improves the corrosion resistance of Mg alloy.
Recently, Ishizaki et al. [32] used magnesium hydroxide/magnesium phosphate compound composite coating for corrosion protection of Mg alloy by a combination process of chemical conversion and steam curing. It was found that the corrosion current density for the coated sample decreased by more than four orders of magnitude as compared to uncoated sample. Zhu et al. [33] and [34] used hydrothermal technique to deposit a protective coating on the Mg alloy. They observed that the conversion coating fabricated using the hydrothermal method gives efficient corrosion protective layer on the Mg alloys. Guo et al. [35] fabricated zinc–aluminum layered double hydroxide bilayer films on aluminum substrates by one-step hydrothermal crystallization method. The advantage of the hydrothermal technique is that the coatings are uniform, compact, and have strong adhesion to substrates and thus, can effectively protect substrates from corrosion. As the hydrothermal crystallization occurs on a 3-dimensional structure, this method can also be used to coat 3-dimensional objects [33].
In the present work, a hydrothermal technique was used to obtain a corrosion protective conversion coating of magnesium hydroxide (Mg(OH)2) on the surface of Mg disks. The idea of Mg(OH)2 coating stems from the fact that if the Mg implant surfaces are already coated with Mg(OH)2, Mg will not react with the fluid in the body and the release of Mg ions and thereby evolution of hydrogen will not take place. The structural and corrosion properties of the hydrothermally coated Mg disks and uncoated Mg disk (control) were studied. The results indicate that the hydrothermally coated Mg disks are corrosion protective compared to uncoated Mg disk which is due to low porosity of the coatings.
Mg disks for the hydrothermal treatment were prepared by cutting Mg (99.9%, Good Fellow, Germany) rod (13 mm in diameter) with a thickness of ∼1.5 mm. Each Mg disk was polished with SiC paper successively up to #1200 grit, followed by ultrasonic cleaning in acetone and isopropyl alcohol for 5 min, respectively. A hydrothermal technique was applied for conversion coatings on Mg disks using deionized water. The deionized water was poured into a hydrothermal reaction vessel, which was made of Teflon lined stainless steel, and filled upto 75% volume of reaction vessel. The Mg disk was kept in the reaction vessel and heated to 160 °C in a furnace. The hydrothermal treatment was carried out for 1, 3, 5, and 7 h. Hereafter, sample 1 represents uncoated Mg disk (control) whereas sample 2, 3, 4, and 5 represent hydrothermal coated Mg disks in deionized water for 1, 3, 5, and 7 h, respectively.
The structural characterizations were performed by X-ray diffraction (XRD) technique. The XRD spectra of the uncoated Mg disk and hydrothermally coated Mg disks were recorded with Bruker AXS (D8 Discover) X-ray diffractometer using the 2 θ– θ scan with Cu Kα ( λ = 0.15405 nm) radiation. The microstructures of the samples were recorded before and after corrosion process by scanning electron microscopy (SEM, SU8000, Hitachi). The degradation rate of the uncoated and coated Mg disks was studied using hydrogen release rate. For this study, the disks were immersed and kept in the phosphate buffered saline (PBS) solution (137 mmol/L NaCl, 2.7 mmol/L KCl, 8 mmol/L Na2HPO4, and 2 mmol/L KH2PO4) and kept in the solution for 2 weeks at 37 °C in a water bath. Hydrogen gas released due to the corrosion of samples was collected into a burette. The reading of total volume of evolved hydrogen gas was recorded at regular time interval.
DC potentiodynamic polarization measurements were performed in 1× PBS solution using Gamry Potentiostat (R600, Gamry Instruments) with a standard three-electrode configuration. A saturated calomel electrode (SCE) and platinum wire were used as the reference and counter electrodes, respectively. Uncoated Mg disk and coated Mg disks were used as working electrode. The polarization measurements were carried out in the applied potential range of ±300 mV vs. SCE, at a scan rate of 5 mV/s. Corrosion potential ( Ecorr) and corrosion current density ( Icorr) were determined using the Echem Analyst software (Gamry Instruments). The electrochemical impedance spectroscopy (EIS) study was performed in the frequency range of 1–106 Hz under 10 mV amplitude of the perturbation signal.
X-ray diffraction patterns for the uncoated Mg disk and hydrothermally treated Mg disks are shown in Fig. 1. As seen in Fig. 1, the observed diffraction patterns of the Mg disk showed polycrystalline nature. All the observed peak positions of the Mg disk were found to be in good agreement with the hexagonal phase of Mg (JCPDS file No. 035-0821). Besides the typical peaks corresponding to Mg, some other diffraction peaks were also observed in the hydrothermally treated Mg samples. The presence of other diffraction peaks in the XRD patterns of hydrothermal treated Mg samples indicates the existence of other phases. The extra diffraction peaks other than those arising from Mg plate were observed to be due to the formation of Mg(OH)2 during the hydrothermal treatment of the Mg disks. The formation of Mg(OH)2 on the surface of Mg disks during the hydrothermal reaction can be explained on the basis of following reactions:
The observed extra peaks in the hydrothermally treated Mg samples were in good correlation with the hexagonal phase of Mg(OH)2 (JCPDS file No. 075-1527). It is also evident from the XRD patterns that the intensity of magnesium hydroxide's diffraction peaks increases with increasing the hydrothermal treatment time, which suggest that the thickness and crystallinity of Mg(OH)2 coating on Mg disk improve with longer hydrothermal treatment time [34]. The lattice parameters of the hexagonal phase of Mg(OH)2 coating were calculated using the following expression [36]
The SEM images of the uncoated Mg disk and hydrothermally Mg(OH)2 coated Mg disks as a function of the hydrothermal treatment time are shown in Fig. 2. As seen in these SEM images, the surface morphology of the treated Mg samples changes with the hydrothermal treatment time. The insets show the SEM images of the same sample with higher magnification. As evident from these microstructures, the hydrothermally treated Mg samples have different surface morphology compared to the untreated Mg disk (control). It is apparent from the surface morphology of sample 2 (hydrothermal treatment time, 1 h) that the growth of conversion coating has started. In sample 3 (hydrothermal treatment time, 3 h), some signatures of hexagonal structure of Mg(OH)2 can be seen. Further, with the increase in the hydrothermal reaction time (5 h and 7 h), the hexagonal structure of Mg(OH)2 conversion coating is clearly evident in the sample 4 and sample 5. The presence of hexagonal structure of Mg(OH)2 conversion coating in the SEM images, has been also confirmed in the XRD analysis of the hydrothermally treated samples.
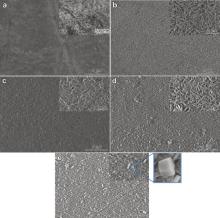 | Fig. 2. SEM images of (a) sample 1, (b) sample 2, (c) sample 3, (d) sample 4, and (e) sample 5 (inset images in each figures show higher magnification). |
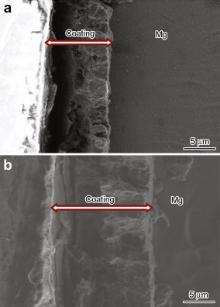
The effect of hydrothermal treatment time on the thickness of the conversion coating on Mg disks was studied using cross section SEM imaging. The cross section SEM images of sample 4 and sample 5 are shown in Fig. 3 as representatives. It is evident from the cross section SEM images that the coatings are dense and uniform on the surface of Mg disk. As evident from the XRD analysis and cross section SEM images, the thickness of the conversion coating increases with an increase in the hydrothermal treatment time. The increase in the thickness with increasing hydrothermal treatment time can be explained on the basis of the effect of temperature and pressure on ionization of water. The ion product of water increases with increasing temperature and pressure. With time the production of hydrogen gas leads to the increase of the pressure in the hydrothermal vessel. This increment in the pressure leads to increasing the degree of ionization of water. This increase in the degree of ionization improves the reaction rate between Mg disk and water [34].
The adhesion of a coating on any surface is a very important factor to determine the quality of the coating. Low quality films could peel off from the substrate and hence are of little importance toward their beneficial application for substrate. The adhesion strength of the conversion coatings on Mg disks was studied according to the standard of American Society for Testing Materials (ASTM) for coatings. ASTM-D3359-02 tape test was chosen to study the adhesion of Mg(OH)2 conversion coating on Mg disk and optical images are shown in Fig. 4, as representative images [37]. In this test, a cross cut patterns through the coating having separation of 1 mm were made on the samples. Fig. 4(a) shows the cross cut patterns before the adhesion test. Fig. 4(b) shows the optical image of the same sample after application and removal of the tape according to the procedure described in the ASTM tape test. As seen in the optical image ( Fig. 4(b)), almost the whole coating was undetached after the removal of the tape from the sample. This indicates that the coating produced by hydrothermal method has strong adhesion on the surface of the Mg disk.
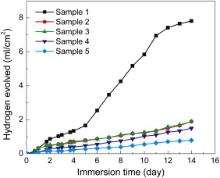
The biodegradability of the untreated and hydrothermally treated Mg disks could be understood in vitro by studying the hydrogen released during immersion in corroding solution similar to body fluid (in this case PBS solution). The study of hydrogen gas evolved during immersion test is significant because 1 mL of H2 evolved corresponds to 1 mg of Mg dissolved, and thus measuring hydrogen gas evolved is equivalent to measuring the corrosion rate of Mg [38] and [39]. Therefore, measurement of hydrogen gas released during the immersion was employed as an important method to compare the biodegradability of the uncoated and Mg(OH)2 coated Mg disks. Fig. 5 shows the hydrogen gas released vs immersion time for the samples in 1× PBS solution maintained at 37 °C using a water bath. As seen in Fig. 5, the volume of hydrogen gas released for uncoated Mg disk is much higher than that of hydrothermally treated Mg disks. Among the hydrothermally treated Mg samples, the volume of hydrogen gas released was lower for the samples treated for longer hydrothermal treatment time. The samples treated for more than 7 h have similar properties compared to that for 7 h. Frankel et al. [40] studied the hydrogen gas evaluation in 0.3% NaCl solution from Mg surfaces during galvanostatic polarization. They observed that the rate of hydrogen evolution was minimum at the open circuit potential, and increased with increasing applied anodic or cathodic current.
The effect of hydrothermal treatment time on the corrosion behavior of Mg disks was studied using potentiodynamic polarization measurements. Potentiodynamic polarization curves for uncoated Mg disk and hydrothermally coated Mg disks were shown in Fig. 6. As seen in this figure, the hydrothermally coated Mg samples show lower corrosion current density compared to uncoated Mg sample. The corrosion potential ( Ecorr) and corrosion current density ( Icorr) of the samples were calculated using the Stern–Geary equation [41].
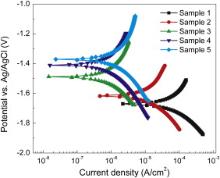 | Fig. 6. Potentiodynamic polarization curves for uncoated Mg disk (control) and hydrothermally coated Mg disks. |
| Table 1. Some important parameters obtained from polarization measurements |
The porosity of the coating is another important parameter for determining the corrosion protective nature of the coating for any substrate. If the coatings are highly porous, the pores of the coatings provide a direct path between the corrosive environment and the substrate leading to severe corrosion of the substrate. The porosity of the conversion coatings produced by hydrothermal treatment on Mg disk was estimated using the following expression [42]:
The corrosion protective nature of the coatings was further analyzed using electrochemical impedance spectroscopy (EIS). The EIS measurements were recorded at the open circuit potential. Fig. 7 shows the Nyquist and Bode plots for the uncoated Mg disk and hydrothermally coated Mg disks. As seen in the Nyquist plot, the real and imaginary part of the impedance increases with decrease in the frequency. As seen in Fig. 7(b), the impedance (|Z|) of the uncoated Mg sample decreases with increase in the frequency and became almost constant over 103 Hz. On the other hand, in the Nyquist plots of the hydrothermally coated Mg samples, the real impedance ( Zre) and imaginary impedance ( Zim) are much higher than that of uncoated Mg sample. In the Bode plot of the coated Mg samples, it is seen that the impedance is higher than the uncoated Mg sample. The higher value of impedance further confirms the corrosion protective nature of the coating for Mg disk [45]. The variations of the phase angle with frequency for the samples are given in Fig. 7(c). The broader peaks observed for the coated Mg samples showed greater compactness of the coating compared to the uncoated Mg sample [46]. The greater compactness and thus low corrosion rate of the hydrothermally coated Mg samples is evident in the phase angle versus frequency plot where the maximum of the phase angle is broad for the coated Mg samples.
A hydrothermal technique was used to grow Mg(OH)2 conversion coatings on bioresorbable Mg disks. The effect of hydrothermal treatment time on the properties of the conversion coating was investigated. The tape test revealed a strong adhesion between the coating and the Mg disk. The hydrothermally treated Mg disks show low degradation than that of uncoated Mg disk. These results suggest that Mg(OH)2 conversion coating by hydrothermal method could provide a potential corrosion protective coating on Mg disk.
This research was financially supported by the National Science Foundation through Engineering Research Center of Revolutionizing Metallic Biomaterials at NCAT. Authors thank Dr. J. Sankar, Dr. Z. Xu, and Dr. Y. Yun, for useful discussion and providing access to their research facilities.
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|
| 18. |
|
| 19. |
|
| 20. |
|
| 21. |
|
| 22. |
|
| 23. |
|
| 24. |
|
| 25. |
|
| 26. |
|
| 27. |
|
| 28. |
|
| 29. |
|
| 30. |
|
| 31. |
|
| 32. |
|
| 33. |
|
| 34. |
|
| 35. |
|
| 36. |
|
| 37. |
|
| 38. |
|
| 39. |
|
| 40. |
|
| 41. |
|
| 42. |
|
| 43. |
|
| 44. |
|
| 45. |
|
| 46. |
|