The antibacterial finishing of cotton-based fabrics has been achieved from quaternary ammonium-based composite particles. This functionalization is based on the simple dilution of a quaternary ammonium cation (QAC) hybrid alkoxide within a sol–gel derived crystalline suspension (CS) of TiO2 in liquid solution. This protocol yields the preparation of QAC–TiO2 (QT) composite sols by using a same CS over a long period of time, and enables an easy regeneration of derived QT sols after quite long aging periods. Composite sols can then be impregnated on various kinds of substrates, including textile fabrics. Fourier transform infrared spectroscopy studies, as well as optical and scanning electron microscopy observations, have been used to investigate chemical and morphological features arising from QT particles. Antibacterial tests have then been performed on so-finished textiles and are discussed in relation to chemical and morphological features. It is shown that this sol–gel route flexibly yields a similarly strong antibacterial activity on cotton-based fabrics against both Gram-negative and Gram-positive bacteria,
Textiles with enhanced resistance against micro-organisms (antibacterial, anti-microbial, antifungal textiles, or else) are becoming an increasingly desirable aim of textile manufacturers [1], [2], [3] and [4]. Finishing treatments are particularly used to prevent three undesirable effects in textiles. The first includes the degradation phenomena like coloring, staining and deterioration of fibers [5], [6] and [7]. Because of their dye degradation potential, even some fungus can be used for removing dye from textile effluent [8]. The second one produces unpleasant odor [9], [10] and [11] and the third effect is the increase of potential health risks [12], [13] and [14]. For instance, most finished textile materials, currently introduced in hospitals and food industries, are designed to protect wearers against the spread of nosocomial infections, inhibit the multiplication of pathogen micro-organisms, and remove the infection sources. In general, antibacterial properties can be imparted to the textile materials by chemically or physically incorporating functional agents onto fibers of fabrics. Among numerous available varieties of antibacterial agents, species containing a quaternary ammonium cation (QAC) have been widely used for decades to disinfect environmental surfaces, for instance in clinical and industrial settings. This particular interest arises from inherent advantages, including a broad activity spectrum against Gram-negative and Gram-positive bacteria, yeasts, or moulds, as well as effectiveness over a wide pH range [15], [16], [17], [18] and [19]. Derived compounds are used in a variety of commercial applications ranging from cosmetic preservatives to hospital disinfectants and sanitizers. QAC-derived compounds, designed to impart antibacterial functionalization on textile fabrics, have already been submitted to many evaluations and commercialized [1], [4] and [20]. Besides, they still make the object of intensive investigations. In the recent literature, many works have, for instance, been proposed to improve the antibacterial finishing procedure and the performances of finished textiles. Deposition of QAC-derived compounds can be achieved through physical techniques on various kinds of supports [21], but the antibacterial finishing of textiles is generally realized through the preliminary chemical preparation of QAC-based liquid solutions, which can in turn be impregnated by using reduced cost techniques such as pad-, spray-, foam-deposition methods, or else [22]. Such liquid solutions can, for instance, be prepared through all-organic chemistry methods [23], [24] and [25].
Another convenient way to impart particular functionalities on textiles from liquid solutions relies on the implementation of sol–gel methods. In these methods, metal ( M) alkoxides ( M N(O R) n) react in liquid solutions through the hydrolysis and polycondensation of alkoxy (O R) groups (where R is an alkyl radical). These reactions yield the formation of an inorganic polymeric network constituted by more or less developed metal–oxygen chains. Some authors have taken advantage of such methods to physically incorporate QAC-based all-organic species within an inorganic silica network deposited on the surface of textile fibers [26], [27] and [28]. These authors reported on good antibacterial performances of so-finished textile fabrics. Organic species can also be deposited through sol–gel routes by using a so-called hybrid approach. In this approach, one (or more) alkoxy groups of a silicon alkoxide precursor are partially substituted by one (or more) organic radicals non reactive to sol–gel reactions. In this approach, sol–gel reactions result in the formation of an inorganic silica network incorporating organic species covalently bonded ( i.e. firmly fixed) to the inorganic network. Over the past decade, so-formed hybrid materials have extensively been studied to formulate coupling agent for fiber/polymer composites or to induce various functionalities on textile fabrics (see reviews Ref. [29] and [30]). However, hybrid alkoxides containing long organic chains are generally poorly reactive to sol–gel reactions [31] and [32]. It is due to the steric hindrance provoked by long organic chains that reduces the chemical interactions between reactive alkoxy groups. When derived sols are deposited on a substrate, their lack of reactivity promotes undesirable dewetting effects at the substrate surface, owing to an insufficiently developed inorganic network that cannot efficiently counteract capillary forces occurring during the sol–gel transformation. Specific and rather complex procedures and protocols have thus to be set up to activate the sol–gel reactions in sols based on such hybrid alkoxides. On the one hand, sol–gel approaches by using a QAC hybrid alkoxide (QACHA) have already been investigated for different (non antibacterial) applications [33], [34] and [35]. On the other hand, some commercialized products intended to impart antibacterial properties on various supports, including textiles, are based on a QACHA diluted in liquid solution [4] and [20], and some very recent works have also reported on the antibacterial functionalization of textiles from QACHA precursors [4] and [20]. However, we surprisingly note that the literature devoted to such functionalization by using QACHAs is quite poor.
In this article, we present a hybrid sol–gel approach yielding QACHA–TiO2 (QT) composite particles intended to impart an efficient antibacterial functionalization on textile fabrics. For industrial applications, it is important (i) to propose simplified elaboration protocols of liquid solutions, which reduce the fabrication costs, and (ii) to optimize the in-time stability of resulting sols, which allows the long term reproducibility of functionalized products derived from a same sol and reduces the need to frequently repeat sol elaboration operations. In a previous work, we proposed a hybrid sol–gel approach meeting such criteria, which was designed to impart a hydrophobic functionalization on naturally hydrophilic cotton-based fabrics [36]. This approach was firstly based on the sol–gel preparation of a liquid suspension containing TiO2 nanoparticles (NPs). As discussed in our previous article [36], it might be considered that this preliminary stage was quite complex, but the derived sol–gel procedure relied on two essential assesses: (i) the resulting TiO2 liquid suspension exhibited an enhanced stability over time and could be used in reproducible conditions for weeks or months, and (ii) a hybrid alkoxide constituted of hydrophobic long alkyl chains (C16) could easily be grafted on TiO2 NPs through a simple dilution of this alkoxide within TiO2 suspensions, just before textile impregnation and without the need of any complex sol–gel formulation or protocol. This latter feature relied on the high surface reactivity of TiO2 NPs, i.e. their ability to graft silicon alkoxides through Ti–O–Si surface bonds formed by hetero-condensation reactions, which led to C16–TiO2 composite particles. In the present article, we study how this grafting method can be extrapolated to the antibacterial functionalization of textile fabrics by using a QACHA precursor. We firstly show how the formulation of so-formed QACHA–TiO2 (QT) composite sols influences the chemical composition of derived species deposited on the surface of silicon wafers, chosen as model supports, together with the morphology of so-obtained coatings. Results are then extrapolated to the antibacterial functionalization of cotton-based textiles.
The functionalization of textile fabrics with QACHA species is based on the preliminary preparation of a TiO2 crystalline suspension through a two-step sol–gel route. A polymeric mother solution (MS) was first prepared by mixing tetraisopropyl orthotitanate (TIPT from Fluka) with deionized water, hydrochloric acid, and absolute ethanol as a solvent, according to our previously published procedure [37]. TIPT concentration in the solution was 0.4 mol/L, and the TIPT/H2O/HCl molar composition was 1/0.82/0.13. The solution was aged at room temperature for two days before use. Then, a crystalline suspension (CS) of TiO2 nano-crystallites in absolute ethanol was prepared from the MS, by using a procedure that has also been developed in our group and previously detailed [38]. Briefly, the MS was first diluted in an excess of deionized water (H2O/TIPT molar ratio of 90) and autoclaved at 130 °C for 6 h. Autoclaving yielded the crystallization of TiO2 NPs diluted in the aqueous medium. An exchange procedure was then performed in order to remove water from the sol and form a CS in absolute ethanol. The final TiO2 concentration in ethanol was 0.24 mol/L. The CS was composed of TiO2 NPs with a diameter of about 5–6 nm, crystallized in the anatase phase, and agglomerated in small polycrystalline aggregates of around 50–100 nm in size [39]. Previous works have shown that CS preparation conditions give rise to very stable sols, i.e. no further particle aggregation takes place over a prolonged aging at room temperature [38]. This stabilization is due to peptization effects induced by the acidic conditions, i.e. electrostatic repulsions between initially formed, positively charged, TiO2 aggregates. Consequently, these suspensions can be stored for several weeks or months before use. Such suspensions have extensively been applied in our group to deposit and study photocatalytic TiO2 films of high homogeneity and optical quality, which can in particularly be deposited on thermally sensitive substrates through low temperature protocols [38] and [39]. In the present work, we study how these suspensions allow envisaging the antibacterial functionalization of textile fabrics through a protocol based on the dilution of a QACHA precursor in the CS.
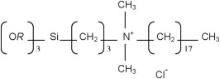
QT sols were formed by using 3-(trimethoxysilyl)propyl-octadecyldimethylammonium chloride (42 wt% in methanol; purchased from Sigma Aldrich) as a QACHA precursor. As shown in the generic representation illustrated in Fig. 1, this precursor basically consists of a silicon methoxide, with three methoxy groups eventually prone to react through hydrolysis/polycondensation or hetero-condensation sol–gel reactions, and a fourth methoxy group which has been substituted by an organic chain containing the QAC. In this structure, the ammonium cation is surrounded by two methyl radicals, a short alkyl chain linked to the silicon atom, and a long hydrophobic C–C chain of 18 carbon atoms. The Cl- ion acts as a counteranion prone to dissociate in liquid solution. In the present work, the pure QACHA precursor was directly added in the CS neither preliminary dilution in any solvent nor any specific sol formulation intended to enhance the sol–gel reactivity of this precursor. The QACHA concentration in the final QT sol was varied from 0 to 0.37 mol/L. Owing to dilution effects induced by the QACHA precursor, the TiO2 concentration in the QT sol concomitantly varied from 0.24 to 0.12 mol/L. In this article, experimental data will be referenced in relation to the QACHA concentration in the QT sols, i.e. these sols will be noted QT x, where x accounts for the QACHA concentration in solution. QT sols were magnetically stirred for 10 min after what they could be used for impregnation. It should be mentioned that mixed sols with the lowest QACHA concentrations exhibited excellent stability over aging in bottle, while QT sols with a QACHA concentration exceeding 0.075 mol/L progressively formed a bi-phased mixture. Since QACHA is directly added in the CS without any water addition, it is likely that this precursor does not undergo any hydrolysis reaction, which can in turn explain that it cannot be efficiently dissolved in the CS to form an even suspension. However, from a practical point of view, this instability had not any detrimental consequence, since the initial mono-phased sol could easily be restored through magnetic stirring even after aging in bottle for several weeks. In the whole range of QACHA concentrations tested here, QT sols could finally be used for several hours in reproducible conditions. The possibility to easily restore mono-phased QT sols (i) arises from the aforementioned good stability of the CS, and (ii) provides a first indication that the QACHA precursor does not undergo any significant chemical evolution in the QT sol owing to its poor sol–gel reactivity. The formation of a bi-phased mixture is probably due to a progressive interpenetration of long organic chains constituting the QACHA, yielding some kind of wax-like physical gel which can easily be re-diluted through magnetic stirring. This interpenetration mechanism, which will be analyzed in next sections, is all the more prone to occur owing to the mutual affinity of hydrophobic organic chains [18]. All these features finally illustrate two specificities of our experimental protocol, which rely on (i) the relative stability of QT sols and the facility to restore them, and (ii) the long term stability of the TiO2 CS, which enables to easily prepare new QT sols by using a same CS over a long period of time.
In preliminary investigations, QT sols were impregnated on (100) silicon wafers (3 cm × 3 cm) by spin-coating (300 μL of sol, spin-speed of 3000 r/min) in ambient conditions. Since chemical or morphological studies of species impregnated on textile fabrics are not straightforward, silicon wafers were used to perform routine characterizations of QT species deposited at their surface. Impregnation of textile fabrics was then performed by using a home-made pad-coating device with a padding speed of 5 m min-1. Routine studies were performed on 2 cm × 6 cm textile samples. For some tests, larger size samples were also punctually impregnated. 65% cotton–35% polyester textiles, furnished by an industrial society, were studied as model samples. These textiles are intended for indoor applications in hospital premises. No additional data can be furnished on these samples for confidentiality reasons. Textile fabrics were functionalized as-received. After impregnation, both silicon and textile supports were dried in air at 110 °C for 10 min to eliminate the residual alcoholic solvent.
Fourier transform infrared (FTIR) spectroscopy was used to analyze chemical features of QT species deposited on silicon wafers. The samples were measured in transmission configuration, in a 4000–300 cm-1 spectral range with a resolution of 4 cm-1, by using a Bio-Rad FTS-165 spectrometer. Spectra of 300 scans were recorded in room atmosphere under dry air sweeping. The spectra were analyzed after subtraction of the bare silicon substrate spectrum. The distribution of QT species on the surface of silicon wafers was analyzed by optical observations by using a Leica DMLM microscope. The thickness of QT coatings deposited on silicon wafers was estimated through ellipsometric measurements by using a Gaertner L116B apparatus operated at a 633 nm wavelength. The morphology of species dispersed on textile fibers was studied by field emission gun scanning electron microscopy (FEG–SEM) with a controlled pressure FEI Quanta FEG 250. The microscope was operated at 3 kV and 120 Pa in secondary electron (SE) mode and at 15 kV and 200 Pa in back-scattered electron (BSE) mode. A Philips XL 30 scanning electron microscope operated at 8 kV was employed for energy dispersive X-ray (EDX) analyses performed on textile fibers. For statistical purposes, EDX measurements were performed on five different places of 2 cm × 2 cm samples.
Determination of the antibacterial activity was focused on the Gram-negative strain Escherichia coli XL1-blue ( E. coli) and the Gram-positive strains Listeria innocua LRGIA 01 ( L. innocua) and Listeria monocytogenes AER 102 ( L. monocytogenes). All strains were stored at -20 °C in brain heart infusion media added with 15 vol.% glycerol. The whole protocol, which is adapted from the ISO 20743-2005 (Anonymous, 2005) standard for the determination of the antibacterial activity on textiles, has been detailed elsewhere [40]. Briefly, after different incubation times at 30 °C, i.e. different contact durations of bacteria on the surface of textile fibers, bacterial cells were removed from the textile and the number of colony forming units (CFU) on trypticase soy agar was counted and expressed relatively to the weight of the textile sample in log(CFU/g) units. For each tested strain, a 10 cm × 20 cm textile sample was impregnated with a QT sol of fixed composition. Small pieces were then cut from the impregnated sample to perform antibacterial tests. Two textile pieces were simultaneously assessed for each tested incubation time. Bacterial evolution kinetics were then studied by plotting log(CFU/g) as a function of the incubation time and the antibacterial activity ( A) of the finishing treatment was calculated after 24 h of incubation according to A = ( C24 - C0) - ( T24 - T0), where C and T represent the numbers of bacteria counted after removal from control (untreated) and treated textiles, respectively, and “24” and “0” account for time of incubation (in hours) for each condition. According to the ISO 20743-2005 standard, the finishing treatment is considered to be active when the antibacterial activity exceeds a value of 2.
Preliminary spin-coating depositions on silicon wafers provided some additional insights in the poor sol–gel reactivity of the QACHA precursor. Deposition of the pure (undiluted) liquid precursor yielded a thick paste on the substrate which could easily be removed through a simple ethanol washing. When the precursor was diluted in ethanol in the absence of TiO2 NPs, derived coatings were observed to dewet the substrate. The most concentrated sols yielded large denuded substrate areas, while for the lowest sol concentrations, any deposited matter could no longer be observed at the substrate surface. In this latter case, it is supposed that all precursor species have been ejected from the substrate through centrifugal forces during the spin-coating procedure in liquid state. These observations depict different detrimental behaviors arising from the absence of efficient sol–gel reactions, i.e. the insufficiently developed Si–O–Si chains formed by the QACHA precursor through hydrolysis/polycondensation reactions and/or the absence of Si–O–Si bonds between QACHA species and the native silica layer present on the surface of the silicon substrate. As explained in introduction, it is expected that QACHA species can be grafted on the surface of TiO2 NPs, similarly to what is observed in the case of C16 ones [36], which can significantly influence their fixation at the substrate surface. These features can in turn be analyzed on the basis of FTIR studies. Fig. 2(a)–(c) shows the FTIR spectra of silicon wafers impregnated from QT sols of various QACHA concentrations. In the low wavenumber range, two bands at around 340 and 440 cm-1, and a large shoulder growing on the high wavenumber side of the 440 cm-1 band, correspond to TO and LO vibration modes of Ti–O–Ti bonds in TiO2 NPs, respectively [41]. As illustrated in Fig. 2(a) for a QT0.008 coating, no additional band could be observed in the low wavenumber range of spectra derived from QT sols of very low QACHA concentration. Further increase in the QACHA concentration yielded the progressive emergence of new bands in the low wavenumber spectral range. It is illustrated in the spectra of Fig. 2(b) and (c) for QT0.08 and QT0.37 coatings, respectively. Several bands can particularly be appreciated in the case of the most concentrated QT0.37 sol ( Fig. 2(c)), which were also observed in the spectrum of the pure QACHA precursor (data not shown). A strong band at 1075 cm-1 and a weaker one at 1090 cm-1 are assigned to methoxy groups of the precursor [42]. Bands at 1465 and 720 cm-1 are assigned to scissoring and rocking vibration modes of methylene (CH2) groups, respectively, which constitute long organic chains of the QACHA precursor [43]. Corresponding asymmetrical and symmetrical stretching modes were depicted by intense bands at 2920 and 2850 cm-1, respectively (data not shown). Interestingly, a very weak band is observed at 975 cm-1. A weak band assigned to quaternary ammonium cations is generally reported to grow in the 990–950 cm-1 spectral range [44], [45], [46] and [47]. Its exact location depends in turn on the nature of chemical radicals linked to the QAC. It is concluded, therefore, that the 975 cm-1 band observed in Fig. 2 depicts the presence of QACs on the surface of our samples.
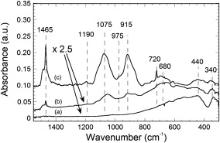 | Fig. 2. FTIR spectra in the low wavenumber spectral range for a silicon substrate impregnated from QT0.008 (a), QT0.08 (b), and QT0.37 sol (c). Absorbance units have been multiplied by a factor 2.5 in the spectra of Fig. 2(a) and (b). |
Spectra of Fig. 2(b) and (c) also indicate several spectral features which were not observed in the spectrum of the pure QACHA precursor. Compared to the pure precursor, the 1075 cm-1 methoxy band is significantly broadened and exhibits two shoulders on its low and high wavenumber sides. These features can partly be attributed to a trans-esterification reaction promoted by ethanol used as solvent, i.e. the partial replacement of methoxy groups by ethoxy ones. Accordingly, most intense bands of a silicon ethoxide are located at 1080 and 1100 cm-1 [48]. Thus, the broadened band at 1075 cm-1 can partially depict a combination of remaining methoxy groups and newly formed ethoxy ones. For previously mentioned reasons, alkoxy groups of the QACHA precursor can hardly develop Si–O–Si polymeric chains. However, adjacent groups of a same QACHA molecule, or groups constituting vicinal molecules, may locally undergo condensation reactions yielding the formation of small size Si–O–Si cyclic species. Such cyclic species are commonly depicted by IR bands at 1110 and 1025 cm-1 [49], [50] and [51]. These bands may in turn also participate in the broadening of the 1075 cm-1 methoxy band. The spectra of Fig. 2(b) and (c) also depict an intense band located at 915 cm-1. Such a band has previously been assigned to non-bridging free broken Si–O– bonds, which are in turn representative of very small (SiO) x clusters (with x less than 6) [52]. All these observations confirm that, when alkoxy groups of the QACHA precursor can eventually undergo in a very limited extent some sol–gel reactions, any significant development of a polymeric network constituted of Si–O–Si chains formed through hydrolysis/polycondensation reactions does not occur. Finally, spectra of Fig. 2(b) and (c) illustrate the appearance of a rather broad band growing at around 680 cm-1, which induces a marked deformation of the Ti–O–Ti LO shoulder observed in Fig. 2(a). This band does not appear in the spectrum of the pure QACHA precursor. Such a band has previously been observed in the case of C16–TiO2 sols [36]. It has been attributed by others to the formation of Ti–O–Si bonds, when titanium ions exist in six-fold coordination [53], as it is the case in our TiO2 NPs. It is inferred that this band depicts the grafting of QACHA species on the surface of TiO2 NPs through hetero-condensation reactions. Since TiO2 NPs in the CS are diluted in ethanol, ethoxy groups are expected to be formed on their surface. Owing to the strong reactivity of titanium alkoxide to hydrolysis reactions [54], ethoxy groups can in turn rapidly react with atmospheric humidity during the deposition procedure, leading to Ti–OH pending bonds on the surface of TiO2 NPs. Finally, these bonds are likely to undergo hetero-condensation reactions with alkoxy groups of the QACHA precursor, leading to Ti–O–Si bonds at the TiO2 particle surface, i.e. the deposition of QT composite particles at the substrate surface. Let us recall that, when diluted in ethanol in the concentration ranges illustrated in Fig. 2(a) and (b), but in the absence of TiO2 NPs, QACHA species totally dewetted the substrate and no IR spectrum could be acquired. This observation clearly shows that hetero-condensation reactions occurring on the surface of TiO2 NPs play a beneficial role in the uniform fixation of QACHA species at the substrate surface.
IR spectra of Fig. 2 also provide additional insights in mechanisms involved in the fixation of QACHA species. Globally, these spectra show that increasing the QACHA concentration in QT sols induces a growing amount of QAC-derived species fixed at the substrate surface. This feature is also illustrated in Fig. 3, which shows intensity variations of the 2920 cm-1 CH2 band of the QACHA precursor as a function of its concentration in solution. This figure indicates that the band intensity increases even faster than the QACHA concentration in solution, following some kind of parabolic evolution. This observation is supported by intensity variations of the QACHA-derived bands illustrated in Fig. 2(b) and (c). These figures show that increasing the QACHA concentration from 0.08 to 0.37 mol/L promotes a dramatic enhancement in the intensity of related bands, while intensity enhancement of the 680 cm-1 Ti–O–Si band appears extremely weak. This feature indicates that increasing the QACHA amount in this concentration range does no longer promote further significant hetero-condensation reactions on the surface of TiO2 NPs. Let us note that, for samples illustrated in Fig. 2(a) and (b), the QACHA/TiO2 molar ratio in QT sols is equal to or less than 0.3, while this ratio equals 3 for the sample illustrated in Fig. 2(c). These data indicate a considerable excess of QACHA in the latter case. Thus, spectral features illustrated in Fig. 2 and Fig. 3 probably depict a bi-regime behavior, which in turn depends on the relative amount of QACHA in QT sols. Below a certain QACHA concentration threshold, fixation of derived species at the substrate surface essentially relies on a grafting mechanism involving hetero-condensation reactions on TiO2 NPs. This chemical grafting is expected to favor an efficient fixation of QACHA species on the surface of TiO2 NPs. Above this concentration threshold, it is inferred that the surface of TiO2 NPs is saturated with Ti–O–Si bonds, in such a way that so-grafted QACHA species shield the TiO2 surface. Thus, QACHA species in excess can no longer chemically react at the TiO2 surface. They are only expected to be fixed at the substrate surface through an interpenetration of their long organic chains with those of chemically grafted species, i.e. a physical mechanism yielding a wax-like coating.
Morphological studies were routinely performed on silicon wafers coated with QT sols. It is important to note that morphology features described hereafter are representative of the whole substrate surfaces. As mentioned in the experimental section, deposition of a pure CS on silicon wafers yielded continuous TiO2 films of high homogeneity and optical quality. A similar appearance was evidenced when the silicon wafers were impregnated from QT sols with a low QACHA concentration of 0.01 mol/L or less (data not shown). Further addition of the QACHA precursor in the CS provoked significant morphology changes, which could be separated in two distinct QACHA concentration ranges typically illustrated in Fig. 4(a) and (b). Fig. 4(a) shows the optical micrograph of a QT0.08 coating. In this moderate QACHA concentration range, QT sols yielded lace-like coatings, where continuously coated areas of the substrate coexisted with denuded areas (see bright regions in Fig. 4(a)) with a characteristic dimension of some tens of microns. Morphological features illustrated in this image probably depict some partial dewetting effects promoted by capillary forces which develop during the post-deposition drying stage. As explained in introduction, in traditional sol–gel protocols by using sufficiently reactive alkoxides, the formation of strong chemical bonds through efficient hydrolysis/polycondensation reactions counteracts capillary forces and prevents dewetting. Since the QACHA precursor grafted on TiO2 NPs cannot undergo significant hydrolysis/polycondensation reactions, partial dewetting effects are unavoidable in our present conditions. However, the micrograph of Fig. 4(a) shows that coated areas remain rather uniformly distributed at the substrate surface. Let us recall once again that, in the absence of TiO2 particles, QACHA species deposited in similar concentration conditions were observed to nearly totally dewet the substrate. It is concluded, therefore, that QT composite particles can be stabilized at the substrate surface, similarly to what occurs in the case of pure CS films. As explained in our previous article [36], during the post-deposition solvent evaporation, Ti–OH pending bonds formed at the TiO2 particle surfaces can react through hetero-condensation reactions with Si–OH pending bonds of the silica layer naturally present on the surface of the silicon wafer. The resulting formation of Ti–O–Si bridging bonds enables in turn the uniform fixation of QT particles at the substrate surface. This mechanism should thus compete with hetero-condensation reactions involved in the grafting of QACHA species on the surface of TiO2 NPs during the deposition procedure.
Further increase of the QACHA concentration in QT sols yielded new morphology changes. It is illustrated in Fig. 4(b) for a QT0.37 coating. In such conditions, the substrate is rather continuously coated but, as illustrated in Fig. 4(b) by color contrasts arising from optical interference effects, the coating exhibits strong local thickness variations. Ellipsometric measurements indicated an average thickness value of around 1 μm. Local thickness variations are, here again, probably due to surface tension effects in relation to an insufficiently developed oxide network. As previously discussed, we infer that the deposition of QT sols of strong QACHA concentration mainly relies on the physical interpenetration of organic chains. It is thus concluded that morphology evolutions illustrated in Fig. 4(a) and (b) depict (i) a deposition mechanism essentially involving QT composite particles in the case of sols with a sufficiently low QACHA concentration, and yielding lace-like coatings, and (ii), in the case of more strongly concentrated sols, a deposition mechanism where the physical fixation of QACHA species in excess becomes preponderant, which yields continuous wax-like coatings. However, preliminary tests also indicated that the impregnations of textile fabrics from the different QT sols promoted an undesirable stiffening of the textiles, irrespective of previously discussed morphologies. It is important to note that such undesired effects are rarely discussed in the literature devoted to the sol–gel functionalization of textile fabrics. In the present case, stiffening was attributed to an excessive amount of impregnated matter. Further experiments were thus performed with QT sols additionally diluted in ethanol. Fig. 4(c) and (d) show optical micrographs of silicon wafers impregnated with the sols illustrated in Fig. 4(a) and (b), which have been additionally diluted by a factor 4 (QT0.02 sol) and 6 (QT0.06 sol), respectively. On the one hand, Fig. 4(c) indicates that dilution of the QT0.08 sol by a factor 4 induces significant morphology changes, from a lace-like coating to a discontinuous island-like coating. This observation probably depicts that an additional dilution in ethanol reinforces dewetting effects. However, the micrograph of Fig. 4(c) also shows that QT composite particles are still uniformly dispersed at the substrate surface. On the other hand, Fig. 4(d) indicates that dilution of the QT0.37 sol by a factor 6 does not induce significant morphology changes. As deduced from interference color observations, the resulting coating is still observed to continuously coat the substrate surface with marked local thickness variations. In these conditions, ellipsometric measurements indicated an average coating thickness of around 100 nm, which showed that dilution in ethanol yielded a strong reduction in the amount of impregnated matter.
Fig. 5(a) and (b) shows the FEG–SEM images of textile fibers impregnated from previous QT0.02 and QT0.06 sols, respectively. A rather rough surface can be appreciated in uncoated areas of the fibers (see dotted arrows). This roughness is characteristic of the cotton fibers, which are the main component of the textiles studied here. Topographic contrasts in SE mode (see full line arrows in the main images) and bright areas arising from chemical contrasts in BSE mode (inserts) enable to unambiguously evidence the presence of species impregnated on textile fibers. Morphological features illustrated in these images are in fairly good agreement with corresponding ones illustrated in Fig. 4(c) and (d) for silicon wafers. Accordingly, the FEG–SEM images of Fig. 5(a) and its insert depict an island-like coating where coated areas with a characteristic dimension of some micrometers are rather uniformly distributed over the different fibers, while the FEG–SEM images of Fig. 5(b) and its insert show that the textile fibers are more continuously (but not totally) coated. Characteristic morphologies illustrated in Fig. 5 were repeatedly observed in different places of the textile samples, which demonstrated that both QT sol formulations yielded a homogenous impregnation of the textiles, irrespective of mechanisms involved in the fixation of QAC-derived species. Impregnations from QT0.02 and QT0.06 sols finally enabled us to totally preserve the hand feeling of the textile, i.e. its softness and smoothness, as well as its visual aspect. It is thus concluded that an island-like morphology in the former case, and a significant reduction in the thickness of the wax-like coating in the latter, cause in turn the good hand feeling of textiles impregnated from such sols.
The antibacterial efficiency of textiles impregnated from QT0.02 and QT0.06 sols was assessed against the Gram-negative E. coli and Gram-positive L. innocua and L. monocytogenes bacteria. Preliminary tests indicated that control (uncoated) textiles, as well as textiles impregnated with only TiO2, did not exhibit any antibacterial behavior. It is illustrated in Fig. 6 in the case of the L. innocua strain. For both textiles, the bacteria follow rather similar evolutions leading to an increasing number of colonies by approximately 3 log(CFU/g) after 24 h of incubation. On the one hand, these observations suggest that textile fabrics, especially those made from natural (cotton-based) fibers, can provide excellent conditions for the growth and proliferation of bacterial cells. On the other hand, they indicate that TiO2 NPs also allow the development of biological cells, which is in agreement with previous works performed in our group showing the biocompatibility of sol–gel derived TiO2 thin films [55]. Actually, it has been shown by other authors that TiO2 NPs are naturally not antibacterial and can only exhibit a bactericide effect when exposed to ultraviolet (UV) light owing to their photocatalytic activity [56]. In a previous work, we showed that TiO2 films deposited through a low temperature procedure from the CS exhibited strong photocatalytic activity under UV light exposure [38]. In addition to a photo-bactericide effect, this activity can also alleviate the antibacterial functionalization by promoting a photocatalytic decomposition of QAC-derived organic species, or even degrade the surface of textile fibers. All these features arising from a UV exposure should be the object of further investigations. However, these features are out of the topics of the present work since, as mentioned in the experimental section, presently studied textiles are intended for indoor applications where UV light is necessarily limited for safety reasons.
 | Fig. 6. Evolution kinetics of the L. innocua bacteria on the surface of a control (uncoated) textile (- - - -), and a textile only impregnated with TiO2 NPs (___). |
Evolutions of the three model bacteria studied in this work are illustrated in Fig. 7(a)–(c) for control textiles and textiles impregnated from a QT0.06 sol. The three strains exhibit very similar evolutions. On the one hand, the number of colonies increases by approximately 3 log(CFU/g) after 24 h of incubation on control textiles. On the other hand, for the three tested strains, the number of cells decreases by approximately 5 log(CFU/g) after a short incubation of 4 h, yielding a log(CFU/g) value of zero, and it does not increase again over more prolonged incubations. In a previous work devoted to the antibacterial activity of textiles impregnated with Ag° NPs, we mentioned that, while a short incubation duration of 4 h yielded a quasi total elimination of the L. innocua strains, further incubation induced a new increase of the log(CFU/g) value by approximately 2.5, showing that some cells could survive after a short incubation and undergo further development over prolonged incubation [57]. In contrast, data illustrated in Fig. 7 demonstrate that no more cultivable bacteria are detected after 4 h incubation. Since TiO2 NPs are not antibacterial, we conclude that the antibacterial activity of textiles studied here can only be caused by impregnated QAC species. Interestingly, bacteria incubated on textiles impregnated with a QT0.02 sol followed exactly the same kinetics of inhibition than those incubated on textiles impregnated with a QT0.06 sol (data not shown), yielding very comparable antibacterial activities. These activities, according to the definition based on the ISO 20743-2005 standard, are illustrated in Fig. 8 for the three tested bacteria. Similarly strong activities ranging between 7.5 and 7.9 are deduced from these analyses, irrespective of the strain nature or QT sol formulation. These activities greatly surpass the value of 2 usually accepted, according to the ISO 20743-2005 standard, to define an active textile. Besides, very small error bars illustrated in Fig. 8, which depict measurements performed on different pieces of a same sample (see Section ), confirm the homogenous impregnation of textiles from our QT sols.
 | Fig. 7. Evolution kinetics of bacterial strains at the surface of a control textile (□) and a textile impregnated from a QT0.06 sol (▪): (a) E. coli, (b) L. innocua, (c) L. monocytogenes. |
It is well admitted that the antibacterial action of QAC species relies on electrostatic interactions arising from the positively charged ammonium cation [1], [17], [18], [19] and [22]. The most referenced mechanism involves a binding of the ammonium cation to the negatively charged bacterial cell wall and subsequent disrupting of the cytoplasmic membrane, which permits the release of potassium or other vital constituents and results in rapid death of the bacterial cell. This action does not necessitate permeation and only involves a contact with the membrane surface [19]. In such a mechanism, the QAC remains intact during cell inactivation and entirely retains its antibacterial ability [1]. Some alternative electrostatic mechanisms have also been envisaged, such as an ion exchange between the positively charged QAC and cations present within the bacterial membrane [19]. These latter cations are thus relieved of their essential role in charge neutralization of the membrane and are free to diffuse out. It causes a loss in the membrane integrity and yields again the bacterial death. Among various aspects which can influence this antibacterial efficiency, many authors have also pointed out the important role of alkyl chains linked to the QAC [17], [18], [19] and [58]. This role relies on the length of alkyl chains, which determines their hydrophobicity. It is generally reported that long alkyl chains with enhanced hydrophobicity promote a penetration of the QAC through the hydrophobic bacterial membrane, which enhances again its antibacterial activity. However, some authors have mentioned that this effect follows a parabolic relationship between antibacterial properties and alkyl chain lengths, medium length chains ( i.e. C6–C8) being the most effective [18] and [19]. Yudovin-Farber et al. [18] proposed an explanation based on the mutual sticking of too long alkyl chains, owing to hydrophobic interactions, which would reduce the QAC antibacterial activity.
In the present work, it could be expected that textiles impregnated from QT0.06 sols would exhibit a greater antibacterial activity than those impregnated from the QT0.02 sol. Indeed, according to the sol compositions, a greater amount of active species is supposed to be impregnated from the QT0.06 sol. However, our present tests suggest that both sols yield very similar antibacterial activities, and no bacteria can develop on the surface of functionalized textile after 4 h incubation. As discussed before, according to the excess of QACHA in the QT0.06 sol where the QACHA/TiO2 molar ratio equals 3, QT species are supposed to form a wax-like coating. Thus, only species present at the external surface of such a coating are expected to undergo electrostatic interactions with the bacterial membrane, and they probably shield the activity of deeper QACHA species. In such conditions, and despite a greater amount of deposited active species, only a reduced fraction of these species can participate in the antibacterial activity. In contrast, for a QT0.02 sol where the QACHA/TiO2 molar ratio equals 0.3, QACHA species are essentially supposed to be chemically fixed on the surface of TiO2 NPs. In such conditions, a larger fraction of these species can interact with the bacterial membrane and participate in the antibacterial activity. Furthermore, the FEG–SEM images of Fig. 5 show that the QT0.02 sol yields lower fiber coverage than the QT0.06 one. At least for textiles coated with the QT0.02 sol, areas of the fiber surfaces which are not coated with active species should therefore favor a certain bacterial development similarly to what is observed on control textiles. However, no bacterial development has been observed after 4 h incubation for textiles impregnated with both kinds of QT sols. This feature has not been explained yet. It may suggest that bacteria preferentially adhere on coated areas of the fiber, or again some kinds of long-range electrostatic interactions between active species and the bacterial membrane.
We have also observed that our finishing treatment yields a similarly strong activity against Gram-negative and Gram-positive strains. Mahltig et al. [27] have recently reported on the antibacterial functionalization of textiles through a QAC-based sol–gel approach and mention that, compared to a Gram-positive strain, this approach leads to a lower efficiency against a Gram-negative one. In this approach, all-organic QAC species were embedded within a sol–gel derived inorganic matrix. It has also been reported that a lower efficiency against Gram-negative strains may be attributed to the fact that, compared to Gram-positive ones, the former strains exhibit a more complicated and denser cell wall structure more prone to resist to the antibacterial action [22] and [58]. However, this assumption does not clearly explain why, in our previous work [57] and that published by Mahltig et al. [27], textiles impregnated with silver particles exhibited a better activity against a Gram-negative strain. We should also point out that the length of alkyl chains linked to the QAC in our present work (C18) greatly excesses the optimal length reported by other authors to promote best antibacterial activities of QACs [18] and [19]. However, in these works, the antibacterial efficiency of QAC-derived species was assessed either in liquid medium (determination of the minimal inhibitory concentration) or when active species were embedded within an organic matrix. Compared to aforementioned works, in the present study, antibacterial assessments have been performed in dry conditions, and active species were not embedded in an organic or inorganic matrix but were present at the fiber surface in the form of QT composite particles. Accordingly, other authors recently reported on the antibacterial functionalization of textile fabrics with composite particles formed from C18 QAC-derived species, and these authors mentioned similarly strong activities against both Gram-negative and Gram-positive strains [3]. It is thus concluded that such a composite particle configuration on the surface of textile fibers is particularly favorable to impart an efficient antibacterial activity on textile fabrics.
Beside an optimization of properties which determine their functionality, i.e. an antibacterial functionality in the present work, finishing treatments have to fulfill additional criteria in order to be considered for practical uses. In particular, the treatments should exhibit sufficient fastness and strength in order to withstand friction damages and washing cycles. Fastness and strength of the finishing treatment in turn depend on the nature of bonds formed at the textile fiber surface, e.g. covalent bonding or physical attachment. In this preliminary work, we mainly focused on feasibility studies intended to (i) assess the potential of our antibacterial protocol, and (ii) study mechanisms involved in this protocol. No particular effort has been made to strongly attach QAC-derived species on textile fibers. However, in the present state, a simple test already described in our previous papers [36] and [57] provided first insights in the fastness and strength of our finishing treatment. For this test, textile samples impregnated from a QT sol were immersed in water, and then exposed for various durations to ultrasonic agitation in a conventional ultrasound bath. The samples were then pressed against a Teflon roller and subsequently dried for 10 min at 110 °C to remove water eventually impregnated within textile fibers during ultrasonication. They were then characterized through weight measurements and EDX analyses. In order to improve the accuracy of these characterizations, the tests were performed from the QT sol showing the greatest QACHA concentration studied in this work, i.e. a QT0.37 sol. For comparison, a pure QACHA sol of 0.37 mol/L concentration (Q0.37) was also tested in the absence of TiO2 NPs. Weight measurements performed before ultrasonication showed that, compared to an impregnation from the Q0.37 sol ( Fig. 9(a)), impregnation from the QT0.37 one yielded a more important matter pick-up ( Fig. 9(b)), i.e. the amount of impregnated matter normalized to the bare textile sample was about 15 wt% for the Q0.37 sol, while it appeared to be around 30% greater for the QT0.37 sol. This greater quantity of impregnated matter cannot be due to the additional presence of TiO2 NPs, since an estimation based on the QACHA and TiO2 weight concentrations in both sols roughly indicates that TiO2 NPs can only contribute to a 5% enhancement in the weight of impregnated matter. This observation indicates a new benefit arising from the use of TiO2 NPs, which favor an enhanced adhesion of QACHA-derived species on the surface of textiles fibers.
For both studied sols, Fig. 9 shows that a 10 min ultrasonication yields a significant loss of impregnated matter, after what quite reduced matter losses are observed over a prolonged ultrasonication of 20 min Fig. 9(a) firstly indicates a somewhat good adhesion of QACHA species on textile fibers, even in the absence of TiO2 NPs, since about 30 wt% of the matter initially impregnated on textile fibers remains present after a 20 min ultrasonication. It is not excluded that, owing to their positive charge, QACs favor a certain adhesion through ionic attractions on the surface of negatively charged textile fibers. Such an adhesion mechanism has already been studied for cationic species on wool and cotton fibers [25] and [59]. However, Fig. 9(b) also shows that the presence of TiO2 NPs favors an enhanced adhesion of impregnated species, since about 45 wt% of the matter initially attached to textile fibers remains present after a 20 min ultrasonication. This enhanced adhesion is supported by EDX data illustrated in Fig. 10. EDX measurements were focused on the Si Kα (1.74 keV) and Ti Kα (4.51 keV) peak intensities, which accounted for the amount of QACHA species and TiO2 NPs impregnated on textile samples, respectively. A significant decrease of the Si/Ti ratio over ultrasonication clearly indicates that weight variations illustrated in Fig. 9(b) predominantly derive from the release of QACHA species. As previously mentioned, a 0.37 mol/L concentration of QACHA species in sols illustrated in Fig. 9(a) and (b) probably induces the predominant formation of a wax-like coating where a major part of QACHA species in excess are rather weakly bound at the fiber surface. Thus, Fig. 9(a) and (b) probably depicts the release of such species in excess. However, some more firmly attached species can remain present on the surface of textile fibers after a prolonged ultrasonic treatment, and Fig. 9 indicates that the amount of remaining matter is enhanced in the presence of TiO2 NPs, which provides new insights in the favorable role played by such NPs. It is supposed that, while QACHA species deposited from QT sols are efficiently grafted on TiO2 NPs, Ti–OH pending bonds present on the surface of these NPs can also react with OH groups present in the cellulosic component of cotton fibers to form –Ti–O–C– bonds. According to this assumption, at least some QT composite particles may be efficiently bonded on cotton fibers, similarly to what is expected to occur through hetero-condensation reactions with the native silica layer present on the surface of silicon wafers. Such an attachment mechanism, which has already been proposed by others for silica particles fixed on cotton fibers [3], would in turn induce relative fastness and strength of our finishing treatment, i.e. an enhanced resistance to the ultrasonication tests. However, it is known that oxygen bridging bonds formed on cellulose are instable and can be readily hydrolyzed in moisture conditions [30]. Thus, we cannot exclude that such hydrolysis also contributes to matter losses illustrated in Fig. 9, leading to the release of QT particles. Fig. 10 finally illustrates an important dispersion of EDX data before ultrasonication, and this dispersion appears to be strongly reduced after a prolonged ultrasonication. If we assume that QACHA species in excess are prematurely released during ultrasonication and QT composite particles remain essentially present after a prolonged exposition to ultrasounds, we can finally conclude from the dispersion of EDX data that QACHA species in excess are probably less uniformly dispersed at the fiber surface compared to QT particles.
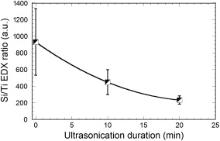 | Fig. 10. Evolution of the Si Kα/Ti Kα intensity ratio deduced from EDX analyses for a textile impregnated from QT0.37 sol and subsequently exposed to ultrasonications of various durations. Error bars arise from five measurements performed on several place of a 2 cm × 2 cm sample. |
In any case, our ultrasonication tests do not account for true practical conditions and we cannot claim that, in the present state, our finishing treatment can withstand intensive friction damages or washing cycles, i.e. can keep their antibacterial activity in daily use conditions. These aspects should be the object of further specific investigations and eventual additional optimizations of our protocol. It is known that epoxy-based hybrid alkoxides play a favorable role in the attachment of sol–gel derived species on cotton fibers [29], [60] and [61]. The beneficial effect of epoxy-derived alkoxides relies (i) on hetero-condensation reactions between functional hybrid alkoxides and epoxy ones, and (ii) on the epoxy ring opening and subsequent covalent binding with cellulosic groups of cotton. This double fixation mechanism finally yields strong bonds between functional species and textile fibers. In a future work, we will study how such effects can be extrapolated to our QT sols in order to reinforce the adhesion of antibacterial species on the surface of cotton fibers.
The antibacterial finishing of cotton-based fabrics has been achieved from quaternary ammonium-based composite particles. Textile functionalization relies on the simple dilution of a QAC-based hybrid alkoxide within a sol–gel derived CS of TiO2 NPs in liquid solution. Assets of this process are firstly based on the fact that (i) the CS exhibits excellent stability over aging, which allows a simple preparation of derived QT sols using a same CS over a prolonged period of time, and (ii) QT sols exhibit in turn a rather good stability over aging owing to their easiness of regeneration. Furthermore, this study shows that the CS promotes an efficient grafting of QACHA species through hetero-condensation reactions on the surface of TiO2 NPs, yielding the uniform deposition/impregnation of QT composite particles. Different types of coating morphologies arise in turn from these QT particles. The formulation of QT sols can then be optimized in order to impart a strong antibacterial activity of so-finished textiles while preserving their hand feeling and visual aspect, irrespective of the coating morphologies arising from the different sol formulations. Textiles impregnated with QT particles exhibit similarly strong antibacterial activities against both Gram-negative and Gram-positive bacteria. Finally, a simple test shows that, even when no particular effort has been made to optimize the attachment of QT particles on textile fabrics, the finishing treatment already exhibits a relative fastness and strength. In future works, new studies will aim at increasing again the adhesion of antibacterial QT particles on the surface of textile fabrics in order to fulfill fastness and strength criteria enabling a preservation of their antibacterial activity in daily use conditions.
This work was performed in the frame of the ACTIPROTEX research project supported by Techtera, the competitiveness cluster for technical and functional textiles based on the Rhône-Alpes Region in France, and by the French government (DGIS). The authors gratefully thank the Rhône-Alpes Region for financial support.
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|
| 18. |
|
| 19. |
|
| 20. |
|
| 21. |
|
| 22. |
|
| 23. |
|
| 24. |
|
| 25. |
|
| 26. |
|
| 27. |
|
| 28. |
|
| 29. |
|
| 30. |
|
| 31. |
|
| 32. |
|
| 33. |
|
| 34. |
|
| 35. |
|
| 36. |
|
| 37. |
|
| 38. |
|
| 39. |
|
| 40. |
|
| 41. |
|
| 42. |
|
| 43. |
|
| 44. |
|
| 45. |
|
| 46. |
|
| 47. |
|
| 48. |
|
| 49. |
|
| 50. |
|
| 51. |
|
| 52. |
|
| 53. |
|
| 54. |
|
| 55. |
|
| 56. |
|
| 57. |
|
| 58. |
|
| 59. |
|
| 60. |
|
| 61. |
|