Rare earth Sm+ ion doped potassium hydrogen phthalate (KHP) single crystal was grown by slow evaporation technique. Single crystal and powder X-ray diffraction analyses confirm the crystalline perfection of Sm+ ion doped KHP crystal. The functional groups of pure and Sm+ ion doped KHP crystals were identified by Fourier transform infrared spectroscopy (FTIR) spectral studies. Thermogravimetric and differential thermal analyses were carried out to study the thermal behavior of the grown crystals. UV–Vis studies explored the optical transmittance of the grown crystals in the entire visible region. The mechanical strength and etching studies were performed to assess the perfection of the pure and Sm+ ion doped KHP crystals. The refractive index and birefringence properties of the grown crystal were analyzed. The second harmonic generation efficiency of Sm+ ion doped KHP crystals was observed by Kurtz–Perry powder test.
Second order nonlinear optical (NLO) materials have recently attracted much attention because of their potential applications in emerging optoelectronic technologies [1]. Materials with large second-order optical nonlinearities, short optical transparency cut-off wavelengths, and stable physico-thermal performance are needed to realize many of these applications. Semi-organic materials have the potential for combining high optical nonlinearity and chemical flexibility of organics with the physical ruggedness of inorganics. Potassium hydrogen phthalate (KHP) crystal is well known for its application in the production of crystal analyzer for long wave X-ray spectrometer [2] and [3]. KHP is a semi-organic material that attracted a considerable attention over few decades. KHP crystals can be easily grown and exhibit interesting dielectric, piezoelectric, pyroelectric, elastic, as well as linear and nonlinear optical properties [4], [5], [6] and [7]. The platelet morphology of KHP crystals has excellent physical properties with good record for long term devices [8] and [9]. Its cleavage faces find applications as substrates in epitaxial technique and KHP crystals are used as substrates for the growth of highly oriented film of conjugated polymers with high nonlinear optical susceptibility [10]. KHP has a well-developed surface pattern on the (010) plane consisting of high and very low growth steps as can be easily observed by means of optical microscopy [11], [12] and [13]. KHP with the chemical formula K(C6H4COOH–COO) belongs to the alkali acid phthalate series, has an orthorhombic symmetry with the space group Pca21 and its lattice parameters are a = 0.9605 nm, b = 1.3331 nm, c = 0.6473 nm and α = β = γ = 90° [14] and [15]. In recent years, researchers have reported a few important investigations on the effect of rare earth ions like Ce4+, Th4+, Eu2+, La3+, Tm3+, Er3+, Dy3+, Tb3+, Gd3+, Pr3+ doped in organic and semi-organic solution grown NLO materials, which enhanced the crystalline perfection and second harmonic generation efficiency [16], [17], [18], [19], [20] and [21]. The ionic radius of Sm+ (0.109 nm) is smaller than that of K+ ion (0.151 nm); hence, Sm+ can either replace K+ or get into the interstitial space, which in both case may create some strain in the lattice. It is well known that doping influences the mechanical, electrical, electronic, optical properties and morphology depending upon the host material and the dopants. In the present work, the KHP was successfully synthesized and the structural and optical properties of the pure and Sm+ ion doped KHP crystals, which are mandatory in view of NLO applications, have been investigated. To have a full understanding about the structure and its nonlinear optical properties of pure and Sm+ doped KHP crystals, single crystal and powder X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), TG–DTA (thermogravimetry–differential thermal analysis), UV–Visible, refractive index, Vicker's hardness, etching, birefringence and second harmonic generation measurements were carried out.
Pure and Sm+ ion doped potassium hydrogen phthalate crystals were grown by slow evaporation method. The potassium hydrogen phthalate (Merck-India) compound was used for both pure and Sm+ ion doped single crystal growth. The KHP salts were further purified by successive recrystallization process. The saturated solutions of pure and 0.1 mol% of Sm+ ion in the form of samarium chloride doped KHP were prepared, according to the measured solubility data. The seed crystals were prepared by spontaneous nucleation from a supersaturated solution and good quality seed crystals were used for the bulk crystal growth. The growth solutions of pure and Sm+ ion doped KHP were saturated at 40 °C. Then, it was heated up 5 °C above the saturation and left it for 1 h under stirring to ensure homogeneity. The saturated solutions were allowed for slow evaporation at 40 °C using a constant temperature bath. The pH values of the pure KHP and Sm+ ion doped KHP supersaturated solutions during the growth period were 3.8 and 3.7, respectively. The transparent pure and Sm+ ion doped KHP crystals were harvested from the supersaturated growth medium in the period of 3–4 weeks, as shown in Fig. 1(a) and (b), respectively.
The grown pure and Sm+ ion doped KHP single crystals were subjected to various characterization techniques. Single crystal and powder XRD analyses were performed to estimate the lattice parameters and crystalline quality of the grown crystals using Bruker Nonius CAD-4/MACH 3 single crystal X-ray diffractometer and Bruker AXSCAD4 diffractometer, respectively. FTIR spectroscopy was effectively used to identify the functional groups of the grown crystals and FTIR spectrum of the sample was recorded by JASCO FTIR 410 spectrometer. TG–DTA analyses were carried out with SII TG/DTA 6300 EXSTAR instrument with a heating rate of 10 °C/min under N2 atmosphere. The optical transmittance spectra of pure and Sm+ ion doped KHP crystals were recorded with Perkin Elmer Lambda 35 spectrophotometer in the range of 190–1100 nm. The refractive indices along b-axis for pure and doped KHP single crystals were analyzed by prism coupling technique. Microhardness measurement was carried out on the grown crystals using Leitz–Weitzler hardness tester fitted with a diamond indenter. The optical birefringence was recorded using modified channel spectrum method (MCS). The relative second harmonic generation (SHG) efficiencies of grown crystals were evaluated by Kurtz powder technique.
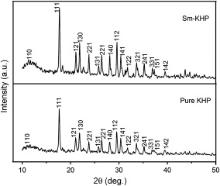
The cell parameters of pure and Sm+ ion doped KHP crystals were collected at room temperature using single crystal XRD analysis. The pure and doped KHP compounds crystallize in orthorhombic crystal system with noncentrosymmetric space group, Pca21. It is observed that the cell parameters of pure KHP crystal presented in are in close agreement with the reported values [15]. The powder XRD patterns of pure and Sm+ ion doped KHP crystals recorded in the 2 θ range of 10°–50° are shown in Fig. 2. The miller indices ( hkl) of the corresponding planes were indexed. It is observed that pure and Sm+ ion doped KHP powder XRD crystal data follow the JCPDS standard data (JCPDS PDF No. 311855).
| Table 1. Crystal data for pure and Sm+ ion doped KHP crystals |
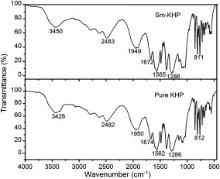
FTIR spectra of pure and Sm+ ion doped KHP crystals recorded in the range of 4000–400 cm-1 by KBr pellet technique are shown in Fig. 3. In the spectra, the characteristic OH stretching peaks occur at 3428 and 3450 cm-1 for the synthesized pure and Sm+ ion doped KHP compound, respectively. The C–C aromatic vibrational peaks are observed at 1485 and 1486 cm-1 for pure and Sm+ ion doped KHP compound, respectively. The characteristic vibrational absorptions of Sm+ ion doped crystal are shifted smaller from pure KHP absorptions. This change could be due to the doping effect of rare earth ion such that Sm+ ion enters into the lattice by replacing K+ ion. From the FTIR spectra, the presence of the functional groups in the pure and Sm+ ion doped KHP compounds has been confirmed. The vibrational frequencies of functional groups presented in were found to be in good agreement with reported values.
| Table 2. FTIR frequency assignments of pure and Sm+ ion doped KHP crystals |
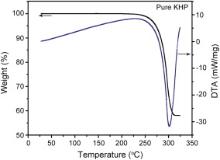
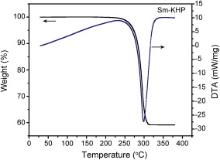
Differential thermal analysis (DTA) and thermogravimetric analysis (TGA) of pure and Sm+ ion doped KHP crystals were carried out in the temperature range upto 400 °C with a heating rate of 10 °C/min in nitrogen atmosphere. TGA and DTA curves of pure and Sm+ ion doped KHP crystals are shown in Fig. 4 and Fig. 5, respectively. The thermal analysis revealed that there is no physically adsorbed water in the molecular structure of Sm+ ion doped KHP crystal. DTA curves of pure and Sm+ ion doped compounds show the sharp endothermic peaks at about 300 °C. TG curve indicates that the samples are stable up to 300 °C. No decomposition till the melting point ensures the suitability of the material for applications in lasers, where the crystals are required to withstand high temperatures.
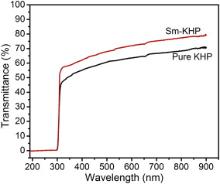
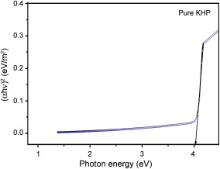
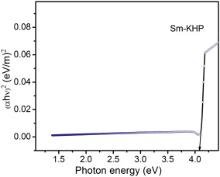
The optical transmittance range and cut-off wavelength of the grown crystals are important factors for optical applications. The optical transmittance spectra were recorded at room temperature in the range of 190–900 nm for pure and Sm+ ion doped KHP crystals, as shown in Fig. 6. The absorption in the entire visible region of the Sm+ ion doped KHP crystal is decreased as that of pure KHP crystal and the cut-off wavelength falls at 300 nm for both crystals. It is observed that the dopants do not change the cut-off wavelengths of the grown crystals. The Sm+ ion doped KHP crystal shows higher transmission than pure KHP and it is expected for the SHG and other optoelectronic applications. The dependence of optical absorption coefficient on photon energy helps one to study the band structure and the type of transition of electrons. The optical absorption coefficient ( α) was calculated from transmittance using the following relation:
The refractive indices for the pure and Sm+ ion doped KHP crystals were calculated theoretically and experimentally along b-axis of grown single crystals by using Metricon prism coupler Model 2010/M in TE mode. The optically polished transparent crystal sample was brought in contact with the base of the prism by means of a pneumatically operated coupling head, creating a small air gap between the crystal and the prism. The prism coupler was used with a He–Ne laser source of wavelength 632.8 nm, a turn-table and a detector. The refraction of the light was observed as knee in the prism coupler result. The experimental values reveal that the measured refractive index values for pure and Sm+ ion doped KHP crystals are 1.486 and 1.5072, respectively.
The optical constants ( n, k) were determined from the transmission ( T) and reflection ( R) spectra based on the following relations [23]:
The refractive index ( n) can be determined from the reflectance ( R) using the following relations [24]
The reflectance in terms of absorption coefficient can be written as
The calculated refractive index values for the pure and Sm+ ion doped KHP crystals are 1.4952 and 1.5138, respectively. The observed refractive indices for pure and Sm+ ion doped KHP crystals by experimental and theoretical estimation are listed in .
| Table 3. Comparison of experimental and theoretical values of refractive index for pure and Sm+ ion doped KHP crystals |
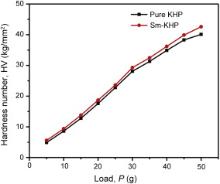
The Vicker's microhardness measurement was carried out on the grown crystals to assess the mechanical property. The static indentations were made at room temperature with a constant indentation time of 10 s for all the indentations. Indentations were made on the (010) plane of a selected crystal using Vicker's microhardness tester. The crystal was mounted properly on the base of the microscope and the selected face was indented gently by the applied loads 5–50 g for a dwell period of 10 s using a diamond pyramid indenter. The hardness number of pure and Sm+ ion doped samples was calculated using the relation,
Since crack initiation and material chipping became significant beyond 50 g of the applied load, hardness test could not be carried out above this load. These results can be attributed to the work hardening of surface layers [25]. A graph plotted between hardness number (HV) and applied load ( P) is shown in Fig. 9. From the figure, it is clear that hardness number (HV) increases with increase of load ( P) for all the crystals and it is also evident that the hardness value for Sm+ ion doped KHP crystals is higher than that of pure KHP.
Etching is the selective dissolution of the crystal, which reveals the crystal symmetry and lattice defects, and it is able to develop some of the features such as growth hillocks, etch pits and grain boundaries on the crystal surface. Etchants described in the literature for organic crystals [26] or prepared by considering the various chemical reactions of the material are proved to be unsuitable to delineate the dislocation presented in the crystal. Etching studies were performed on the (010) plane of pure and Sm+ ion doped KHP crystals. The as-grown pure and Sm+ ion doped KHP crystals were etched with water for 10 s. The etched surface was soaked with a filter paper and examined under an optical microscope. Fig. 10(a) and (b) shows the etch patterns on the (010) plane of as-grown pure and Sm+ ion doped KHP crystals and it produced well defined etch pits.
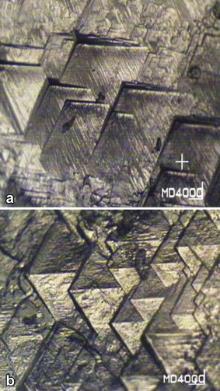 | Fig. 10. Etch pit patterns observed on (010) plane of pure KHP (a) and Sm+ ion doped KHP crystals (b). |
The modified channeled spectral method (MCS) was used for the measurement of birefringence property of the grown crystals and 500 W halogen light source served as the source. The light beam was allowed to pass through a monochromator. A green light of 550 nm was allowed to travel through (010) plane of the grown crystal samples of thickness about 0.076 mm [27]. The birefringence spectra of pure and Sm+ ion doped KHP crystals are shown in Fig. 11. The birefringence values of pure and Sm+ ion doped KHP specimens were calculated using the relation [28]:
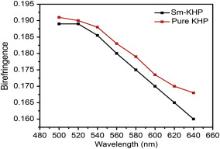 | Fig. 11. Birefringence spectra of pure and Sm+ ion doped KHP crystals. |
The SHG test was performed on the grown crystal samples by employing Kurtz–Perry powder test [29]. The Nd:YAG laser with 1064 nm wavelength was used as the optical source and directed on the powdered sample through a filter. The doubling of frequency was confirmed by the green light emission at 532 nm wavelength. Pure and doped KHP structures are having favorable molecular alignments for high nonlinear optical properties [30]. The SHG signal output voltages of pure KHP, Sm+ ion doped KHP and potassium dihydrogen phosphate (KDP) samples were measured to be 16.5, 33 and 18.5 mV, respectively. Thus it is observed that SHG efficiency of Sm+ ion doped KHP crystal was found to be 1.78 times that of KDP reference crystal. The SHG efficiency of Sm+ ion doped KHP crystals was compared with Ce+ (19 mV), Y+ (25 mV) doped KHP crystals and found higher value for Sm+ ion doped KHP crystal [16] and [31]. Thus, Sm+ ion doped KHP crystals have improved NLO properties compared to pure KHP crystal.
Good quality single crystals of pure and Sm+ ion doped KHP were grown by slow evaporation technique. Single crystal and powder X-ray diffraction studies confirmed the incorporation of Sm+ ion into the lattice of KHP crystal. The presence of functional groups of the grown crystals was confirmed by FTIR spectral analysis. TG–DTA analyses confirmed that there is no decomposition of the crystals upto the melting point. UV–Vis transmittance studies reveal the transparency, cut-off wavelength and band gap energies of the grown crystals. Microhardness studies on the Sm+ ion doped KHP crystal possesses relatively higher hardness values compared to pure KHP crystal. Etching studies on the (010) plane of the pure and Sm+ ion doped KHP crystals confirm the two dimensional nucleation and spreading of layers with the relevant growth mechanism. The optical refractive index and birefringence of the crystal were examined for grown crystals. The SHG output signals were observed on the grown crystals and found that the grown crystals are suitable for optoelectronics applications.
One of the authors (M. Krishna Kumar) acknowledges Council of Scientific and Industrial Research (CSIR), New Delhi, India for providing financial support (Project No. 03(1200)/EMR-II).
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|
| 18. |
|
| 19. |
|
| 20. |
|
| 21. |
|
| 22. |
|
| 23. |
|
| 24. |
|
| 25. |
|
| 26. |
|
| 27. |
|
| 28. |
|
| 29. |
|
| 30. |
|
| 31. |
|