The aim of this study was to investigate the influence of ethylenediaminetetraacetic acid (EDTA) irrigation on demineralization rate of dentine located in the apical third of root canal walls. Teeth were divided into A and B two groups. In group A, all of the teeth was irrigated with EDTA and NaOCl (sodium hypochlorite), followed by cutting the apical third into slices longitudinally to examine the influence of EDTA on different portions of apical third of root canal. In group B, the apical third of a tooth was firstly cut into slices longitudinally, followed by coating the root canal walls with EDTA to
The apical calcification has received much attention as a major reason of the failure of entrance of file to apical in dental treatment [1]. Especially, for pulp diseases and periapical diseases, it is necessary to remove the nerve from the root canal. While the apical calcification will hinder the entrance of root canal. Therefore, the remove of calcified deposition is an essential step for the further treatment.
Root canal treatment has been proven to be an effective way to remove the calcified deposition from the dentine surface of the canal wall [2]. However, the smear layer tends to be created during root canal preparation, which has an adverse effect on the entrance of files in root canal [3], [4], [5] and [6]. Debris of calcified deposition and the organic component in root canal are the major components of the smear layer [3]. Therefore, irrigants that can dissolve inorganic and organic components are frequently used to remove the smear layer.
During root canal cleansing, ethylenediaminetetraacetic acid (EDTA) and sodium hypochlorite (NaOCl) irrigants are generally adopted to remove the calcified deposition and organic component, respectively [3], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17] and [18]. EDTA, known as a chelating agent, is considered to remove the calcified deposition by demineralizing and softening the root canal dentine. To date, there are numerous reports concerning the effect of EDTA on the root canal dentine. Ozdemir et al. [8] investigated the chemical and ultra morphologic effects of EDTA on the root canal dentine, and found different demineralization for young and old dentine. Yilmaz et al. [15] investigated the influence of EDTA solutions on the wettability of root canal dentine and found that the use of low-surface-tensioned EDTA compounds alone or in combination with NaOCl could increase the wettability of root canal dentine. Putzer et al. [18] compared concentration effect of EDTA on the cleaning efficiency of root canal, and found that the increase of concentration can enhance the cleaning efficiency of irrigant. Hulsmann et al. [19] reviewed the demineralization effect of EDTA on chelating agents. However, to the best of our knowledge, there is no systematical investigation on the demineralization of EDTA on different part of dentine located in apical third; especially, the demineralization rate of apical dentine has not been determined. Generally speaking, scanning electron microscopy (SEM) can be applied to observe the size change of root canal. However, since the non-conducting of root canal, it can hardly monitor the size change of root canal wall instantaneously. As a result, instead of obeying a standardized reacting time of EDTA, the clinical endodontics have to estimate a reacting time of EDTA according to their experience.
In this paper, we presented an in-vitro investigation on demineralization effect of EDTA on different portions of apical third of root canals. Furthermore, demineralization rate of apical dentine was calculated by in-situ observation on the widening of root canals vs. the reacting time of EDTA instantaneously.
The Ethical Committee of General Hospital of Shenyang Military Area Command approved the collection and use of extracted teeth for this study. Twenty-six freshly extracted human teeth were treated through the debridement of surrounding soft tissue and debris, followed by disinfecting with autoclaves and placing in saline solution at 4 °C. Then the teeth were assigned to A and B two groups. In group A, teeth were irrigated with EDTA, followed by splitting longitudinally. While in group B, the teeth were firstly split longitudinally into slices, followed by coating with EDTA. The detail of the experiment is as follows.
Group A: A total of twenty-two teeth were collected and four teeth with single root canal were adopted. Crowns of the teeth were removed and the roots were retained. Firstly, root canal preparation was carried out with a Dentsply-Maillefer K-file by a step-back technique; then, the teeth were irrigated with 1.25% NaOCl for 20 times, followed by irrigation with physiological saline to remove the debris through a syringe (25 mm, C–K, Korea) with volume of 10 ml; afterwards, 1.0 ml of the commercial EDTA paste (Meta Biomed Co., Ltd, 19% in concentration) was introduced into the root canal and the treating time was lasted for 1–4 min, followed by irrigation with 5 ml NaOCl (1.25% in concentration) and physiological saline for 20 times to remove the EDTA thoroughly; finally, the root was mounted with epoxy resin, followed by cutting the apical third (the portion from apex to upper end, i.e., from tip to interface between apical third and middle third) into five slices with thickness of 700 μm with a histotome (EXAXT310CP, Germany). Optical microscopy (BX51, Olympus, Japan) was adopted to observe different part of the root canal treated with different time. Scanning electron microscopy (SEM, ZEISS, Ultra Plus, 5.0 kV) was adopted to observe different part of root canal.
Group B: A total of four teeth were collected and one single-rooted tooth was adopted. The step-back technique was also applied in the root canal preparation; then, the apical third was cut into three slices with thickness of 700 μm with an automatic bar lathe; afterwards, the surface and side walls of slices were irrigated with 1.25% NaOCl solution and physiological saline (10 ml per unit time) for 20 times; finally, about 0.06 ml EDTA paste was introduced in the medial wall of the slice directly. Instead of irrigating the medial wall with NaOCl solution and physiological saline, EDTA remained on the medial wall of slice all the time for investigation on the effective influence duration of EDTA on root canal. Optical microscopy was adopted to observe the cross-section of root canal slices instantaneously for about 40 min, and the micrograph of the root canal wall was taken with the time interval of 1 min. Furthermore, SEM was adopted to observe the influence of EDTA on the microscopic morphology of root canal wall in-situ , and the treating time of EDTA was from 20 to 40 min, with time interval of about 5 min.
Finally, X-ray diffraction (XRD) with Cu K α radiation (30 kV, D/max 2000, Rigaku, Japan) was adopted to monitor the changes of root canal dentine before and after the introduction of EDTA on the cross-section of slice for 30 min.
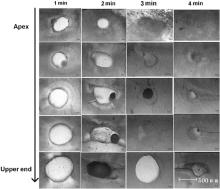
Fig. 1 shows optical micrograph of apical third slices treated with EDTA for different time. Different demineralization rate was found for different part of apical third for the same treated time, i.e., demineralization effect of EDTA on apical third decrease from apex to upper end. Furthermore, the root canal wall was also “dissolved” by EDTA, and the wall at apex is different from that at upper end of apical third. Therefore, EDTA has a demineralization effect on dentine, and the different demineralization rate at different part of apical third is owing to the different amount of EDTA irrigation at different part. It should be noted that different tooth has different size of apical third, and it is impossible to obtain the demineralization rate of dentine by calculating the size change of different root canal vs. reacting time of EDTA.
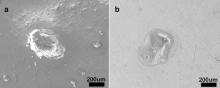
Fig. 2 shows different parts of apical third with the same EDTA treating time of 2 min Fig. 2(a) and (b) are SEM images at apex and upper end of the apical third, respectively. It was found that both the apex and upper end of apical third have irregular shape, and the size of apex is in the range of 319 μm–372 μm, while the size of upper end is in the range of 427–567 μm. In addition, there are obvious double borders of root canal wall for the upper end after the treatment by EDTA. Therefore, EDTA may have a stronger influence on the apex than the upper end of apical third, which is in accordance with the results of optical microscope.
Fig. 3 shows the size of a root canal with reacting time of EDTA via optical microscopy. The light area and darkened area are the apical foramen and root wall, respectively. The size of root canal enlarges with the increase in reaction time. Besides, the size hardly increases when the reaction time exceeds 30 min. Then, demineralization rate of dentine can be calculated by measuring the size change of root canal wall for different reacting time of EDTA.
 | Fig. 3. Optical micrographs indicating the variation of a root canal size with reacting time of EDTA. The light and darkened area are the apical foramen and root wall, respectively. |
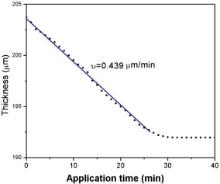
Influence of EDTA on the horizontal linear size of apical foramen with different reacting time was investigated with time interval of 1 min. With the enlargement of apical foramen, the horizontal linear size of apical foramen will increase and the linear thickness of root wall will thereby decrease. Since the linear size of light area and darkened area can change with the reacting time of EDTA, the size dependence of reacting time for apical foramen can be measured from the graph of optical microscope. Then, the decrease of linear thickness root canal wall can be calculated. In the experiment, the horizontal linear size of apical foramen was measured from the one third parts of the photos from bottom. Table 1 lists some of the data with the time interval of 5 min. According to the table, the linear thickness of root canal wall decreased about 2 μm per unit time interval at the first 30 min. Thereafter, the thickness was no longer changed with the increase of time. Furthermore, Fig. 4 shows the thickness of canal wall vs. reacting time of EDTA. The thickness change is nearly stable for the first 25 min, i.e., the demineralization rate of dentine is stable; afterwards, the change slows down from 25 min to 30 min, and it hardly changes when the reacting time exceeds 30 min. According to the figure, the demineralization rate of dentine was calculated to be about 0.439 μm/min for the first 25 min.
| Table 1. Thickness of root canal wall located at apical third with different reacting time of EDTA |
Fig. 5 shows SEM images of root canal with different reacting time of EDTA of 20 min, 25 min, 30 min, 35 min and 40 min, respectively. The size of the apical foramen was measured by comparing the diameter of the root canal, which has been marked in the figure. The sizes were calculated to be 992 μm, 1000 μm, 1009 μm, 1018 μm and 1018 μm for Fig. 5(a)–(e), respectively. Therefore, it can be inferred that the size of the apical foramen can increase within reaction time of 35 min, and the size will be no longer enlarged excess 35 min. In addition, demineralization rate of dentine within 35 min is about 1.8 μm/min. The results of SEM have a slight difference from that of optical micrograph on both of the functional duration and demineralization rate. Furthermore, it can be seen that there was a shadow ring around the root canal, which may hint that EDTA diffused and reacted with the dentine surrounding the root canal wall.
 | Fig. 5. SEM images of size of root canal with different reacting time of EDTA: (a) 20 min, (b) 25 min, (c) 30 min, (d) 35 min, (e) 40 min. |
Fig. 6 shows the partial enlarged drawings of borders between the root canal wall and the shadow ring in Fig. 5, and the reacting time of EDTA in Fig. 6(a)–(e) is 20 min, 25 min, 30 min, 35 min and 40 min, respectively. There is no obvious change in morphology of borders with the duration of reacting time of EDTA, i.e., different reacting time of EDTA only leads to the change of thickness in root canal wall. Besides, it was found that the structure of borders is porous, while the residual dentine is dense. Therefore, ingredient of dentine can be removed during the demineralization process of EDTA, which leads to a porous structure at the root canal wall. Thereafter, EDTA can diffuse into the dentine through the pores and react with dentine surrounding the root canal wall. The demineralization functions of EDTA decrease with the increase in diffusion depth. As a result, the calcified deposition and dentine near the root canal wall with more reaction had pores with larger size, while smaller size would be found in the dentine with the increase in depth of penetration of EDTA, and then the shadow ring around the root canal wall can be formed within the scope of demineralization function for EDTA.
 | Fig. 6. Partially enlarged drawings of borders between root canal wall and shadow ring in Fig. 5, with the reacting times of EDTA: (a) 20 min, (b) 25 min, (c) 30 min, (d) 35 min, (e) 40 min. |
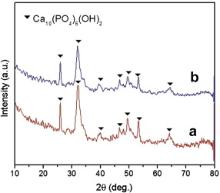
Fig. 7 displays XRD patterns of root canal dentine before ( Fig. 7(a)) and after ( Fig. 7(b)) the reaction of EDTA. All of the diffraction peaks can be contributed to hydroxyapatite (HA). There is no clearly difference between specimen before and after the treatment of EDTA. In addition, intensity of dentine before the treatment of EDTA is a little stronger than that after the treatment, which may be contributed to the EDTA–metal complex attached on the surface of dentine.
To date, there have been numerous biomaterials applied in dentistry [20], [21] and [22]. As mentioned earlier, EDTA is routinely recommended as a chemical irrigant, and the role of EDTA has been clinically investigated intensively [15], [18] and [19]. In our experiment, two approaches were adopted to investigate the demineralization rate of EDTA on dentine. For the first approach (group A), according to our clinical experience and instruction book of EDTA paste (Meta Biomed Co.), the irrigation of root canal with NaOCl is necessary to remove the EDTA after the success of enlarging the root canal. While for the second approach (group B), since it can be hard to observe the demineralization effect instantaneously in clinical implantation, we just investigated the influence of EDTA on dentine instantaneously, and it is unnecessary to remove the EDTA with NaOCl. In this way, the quantitative demineralization rate can be calculated through the measurement of linear size change of root canal. As for the reacting time, the optimal reacting time of EDTA is still controversial [23] and [24]. Therefore, in group A, the reacting time of EDTA is within 4 min to simulate the clinical situation, and the results show that EDTA can effectively enlarge the size of root canal, but with a different demineralization rate. While for group B, in order to establish a systematical relation between the reacting time and size change of root canal, a longer reacting time up to 40 min is adopted. It should be noted that both the optical microscope and SEM were adopted to observe the root canal instantaneously. Owing to the non-conducting of root dentine, the electron may focus during the observation via SEM even if the gold coating has been deposited on the surface of root dentine. As a result, time intervals for SEM observation are greater than that for optical microscope. Interestingly, the results of group B show that demineralization rate based on the optical micrographs is different from that obtained from SEM images. Therefore, it is necessary to study the demineralization mechanism of EDTA in root canal treatment. Fig. 8 shows the schematic routine treatment for root canal calcification. Fig. 8(a) shows a root canal part of which is calcified. As well known, the primary component of calcified deposition is the same as the root dentine, i.e., the hydroxyapatite. Therefore, during the process to remove the HA deposition, it may cause peritubular and intertubular erosion of dentinal tubules, thus reducing the dentine microhardness, consequently causing root fragility [25] and [26]. EDTA is a hexadentate ligand ( Fig. 8(c)), and the demineralization effect of EDTA on dentine lies in its complexing action to metal ions [27], [28] and [29]( Fig. 8(d)). The chelating reaction between EDTA and HA is as follows
where Y is EDTA after the process of dehydrogenation. Ca2+ can be removed from HA after the chelating reaction, which can soften the calcify deposition. As a result, in the clinical root canal treatment, the calcified deposition can be removed effectively and the root canal can be enlarged ( Fig. 8(b)). Then the file can enter the root canal successfully for the treatment of pulp diseases.
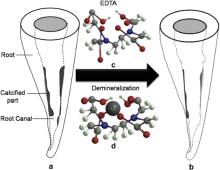 | Fig. 8. Schematic root canal before (a) and after (b) routine treatment of calcification via EDTA; (c) and (d) are structure of EDTA and EDTA–Metal complex. |
In the meanwhile, it should be noted that the chelating of EDTA on HA has no preference on calcified deposition or HA from “normal” dentine of a patient, i.e., the “normal” dentine can also be removed under the reaction of EDTA, leading to the irregular shape of root canal. In this respect, how to define the “normal” dentine and “hyperplasia” dentine is the key point. The “normal” dentine is the healthy body tissue of a patient. In contrast, the “hyperplasia” dentine is the calcified part, which can narrow the root canal and hinder the entrance of the file. Therefore, the reaction time of EDTA plays an important role in removing the “hyperplasia” dentine and avoiding the damage of “normal” dentine. According to results of optical microscope, the effective demineralization time for EDTA is within 30 min, which is in accordance with the effective lasting time provided by manufacturer. However, according to results of SEM with higher resolution, the effective time (35 min), effective scope (the penetration depth of EDTA) and demineralization rate of EDTA are greater than those observed with optical microscope. Furthermore, diffusion of EDTA into the dentine will lead to different degree on softening the calcified deposition and the “normal” dentine, which may lead to the damage in the dentine. Therefore, combined with the results of optical microscope and SEM, the demineralization rate of dentine is remarkable in the first 25 min, the effective treatment duration per unit time should be within 25 min. Furthermore, in most case for a clinical treatment, the introduction of EDTA in root canal for one time is not enough to remove all of the calcified deposition. Therefore, the duration for replacement of EDTA should not exceed 25 min. Additionally, the manual enlargement of root canal with files is also very important in order to shorten the treatment time and protect the “normal” dentine.
Demineralization effect of EDTA on root canal dentine was observed via both ex-situ and in-situ methods. For group A, it was found that EDTA has different influence on different portions of apical third of root canal, and it is less aggressive in the apex when compared to upper end of apical third. For group B, diffusion of EDTA into root dentine will lead to a change in effective duration, effective scope and demineralization rate of dentine. As a result, different demineralization rates were obtained through optical microscope and SEM. Furthermore, the effective treatment duration per unit time should be within 25 min. The investigation can instruct the dentist for a superior implementation of root canal treatment.
The authors gratefully acknowledge the financial support of the project from the Foundation of the Education Department of Liaoning Province (Grant No. L2013285) and Science and Technology Planning Project of Shenyang City (Grant No. F11-262-9-16).
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|
| 18. |
|
| 19. |
|
| 20. |
|
| 21. |
|
| 22. |
|
| 23. |
|
| 24. |
|
| 25. |
|
| 26. |
|
| 27. |
|
| 28. |
|
| 29. |
|