Antibacterial materials play an important role in clinical application, and silver has been known to exhibit strong cytotoxicity towards a broad range of micro-organisms. In this work, the amorphous calcium phosphate with silver substitution (Ag-ACP) was synthesized by chemical precipitation method, and the valence of silver in ACP was adjusted by temperature. The processed Ag-ACP was combined with slightly acidic compounds to form new calcium phosphate cement (CPC). Our results indicate that the valence of silver in CPC was adjusted successfully by chemical precipitation method and heat treatment. X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) results demonstrated that silver ion in CPC-1 and CPC-2 existed in Ag3PO4; after heat treatment of 460 °C, silver became more stable in CPC-3 and CPC-4. Silver in CPC-1 and CPC-2 exhibited better releasing property. After heat treatment at 460 °C, the amount of silver ion released from CPC decreased significantly. Besides, the antibacterial ability of Ag-CPC was adjusted by changing the valence of silver in Ag-CPC. Depending on the low valence of silver and good silver release, CPC-1 and CPC-2 exhibited better antibacterial activity. We believe that this study will motivate the development and applications of antibacterial CPC in bone tissue regeneration.
In clinical application, the wound contamination or postoperative infections are one of the most serious problems in orthopedic surgery, which can lead to severe pain, perhaps loss of bone tissue and even removal of implants. Therapies of infectious diseases, which were considered as a matter of routine earlier, have become increasingly challenging [1], [2], [3] and [4]. A lot of researches had been focused on the impregnation of antibiotics within degradable materials [5], [6], [7] and [8]. In spite of positive therapy results and successful reimplantation in most cases, the risk of bacterial resistance due to low releasing did persist [9], [10] and [11]. It is necessary to figure out new methods to prevent bacterial infections, giving the increasing number of bacterial resistance toward traditional antibiotics. Silver has been used as antibacterial agent in medicine for a long period of time, and silver was applied in medical applications such as silver-coated catheters, antimicrobial bandages, and silver-containing implant coatings [12], [13], [14], [15], [16], [17] and [18]. Most of silver-doped antibacterial agents were based on inorganic materials, such as zeolite, calcium phosphate and silica gel [19], [20], [21] and [22]. Silver-doped delivery carrier was proposed to avoid biofilm growth onto surfaces and/or to release silver ions in a controlled manner to inhibit bacterial proliferation within an infected tissue [23], [24], [25], [26] and [27].
In current clinical application of bone repair, the local delivery of antibiotics in the treatment of postoperative infections was facilitated by poly(methyl meth-acrylate) (PMMA) beads as carriers for drugs [28]. But PMMA beads were not absorbable. In contrast, CPC was not only absorbable but also highly likely to be used as drug carriers in vivo. CPCs, consisting of α- and β-tricalcium phosphate (TCP), had been verified to be useful for osteoconductivity in clinical practices. CPC was first proposed in the middle of 1980s by Brown and Chow [29]. Due to its self-setting performance, easy plasticity, and good biocompatibility, CPC had been commonly used as bone substitute materials and drug carriers in the treatment of skeletal diseases [7], [30], [31], [32], [33], [34] and [35]. Different from the inorganic mineral as silver carrier, CPC consisted of several calcium phosphates and liquid phase. Generally, silver was imported into CPC through liquid phase like silver nitrate (AgNO3), which was a dissolvable source of silver ions [36]. But this method can cause a burst releasing of silver from CPC. And silver ion imported by liquid phase is unstable in the cement matrix, which would reduce its antibacterial ability significantly. Therefore in CPC system, importing silver through liquid phase was not a good choice.
In human bone minerals (apatite), the other ions (Si, Na, Sr, K) shared the same physiological pathway with calcium (Ca) [37]. Ion-doping would cause lattice change due to the different atomic radius with Ca, which altered the solubility of mineral [38] and [39]. CPC were usually produced by acid-based reaction among several calcium phosphate compositions [40], and this reaction was a dissolution and precipitation process. During hydration reaction, the other ions could be doped in the hydration product just like human bone minerals. Ewald et al. synthesized the silver-doped CPC, in their study silver was doped in tetracalcium phosphates (TTCP) at high temperature, which was used as raw hydration material [28]. It was known that the current CPC hydration systems generally need a high-temperature calcium phosphate component such as a-TCP, β-TCP and TTCP, which must be produced by sintering techniques. But according to recent research results [41], [42], [43], [44] and [45], temperature would negatively affect the oxidation/reduction reaction of silver during the formation of silver colloids in heat treatment. These oxidation/reduction reactions would affect the optical density and absorption wavelength of silver colloids. It was also reported that different forms of silver exhibit different bactericidal properties [28]
.In our study, silver was substituted in an Ag-ACP by chemical precipitation method, and the processed Ag-ACP was reprocessed by heat treatment. The change in the valence of silver was characterized by XPS analysis. CPC matrix consisted of Ag-ACP combined with slightly acidic compounds; and all the reactants of CPC were processed under non-calcined synthesis condition. The purpose of this study was to access the effect of silver on this novel hydration system and heat treatment on the antibacterial activity of Ag-CPC. Hydration reaction of CPC was a complex process, different from the single crystal growing process. It was difficult to characterize the valence of silver in hydration product by traditional characterization method. Thus, XPS analysis was introduced to characterize results.
Ag-ACP was synthesized from an aqueous solution of Ca(NO3)2·4H2O, AgNO3and (NH4)2HPO4·12H2O by chemical precipitation method. The concentration of AgNO3was 0.2 mol/L. The deposit was centrifugally separated (LXJ-ⅡB), freeze-dried, and then ball-milled. ACP powder was ground using a planet mill (QM-BP) at a rotation speed of 500 r/min with absolute alcohol as a dispersion medium. Yttria-stabilized tetragonal zirconia balls and polyamide vessels were used during the ground process. In our work, different Ag-ACP was prepared under different reaction temperatures ( Table 1): reaction temperature of ACP-1 was 60 °C; reaction temperature of ACP-2 was 25 °C; and then ACP-1 and ACP-2 were heated at 460 °C to get ACP-3 and ACP-4, respectively.
| Table 1. Component of as-processed Ag-ACP and Ag-CPC |
CPC-1, CPC-2, CPC-3 and CPC-4 (average particle size of 25.6 μm) were prepared by mixing ACP-1, ACP-2, ACP-3 and ACP-4 (average particle size of 7.8 μm) with dicalcium phosphate anhydrous (DCPA) (1:1) respectively with mass ratio of 1:1. Deionized water was used as liquid phase, and the liquid/powder ratio of CPC was 0.4 mL/g. CPC-0 was prepared by mixing ACP without silver and DCPA with deionized water. ACP without silver was synthesized by chemical precipitation method, and the reaction temperature was 25 °C. All commercial chemicals were AR grade in purity. Steel cylindrical molds with an inner diameter of 6 mm and a height of 12 mm were used to prepare cement columns. The cement specimens were stored for setting in an incubator at 37 °C and 97% humidity.
The antimicrobial activity of CPC sample was tested using Escherichia coli ( E. coli , ATCC 23282), which was provided by Institute of Chemical Technology and Light Industry of South China University of Technology. Tryptic Soy Broth (TSB) (Shanghai No. 4 Reagent&H.V. Chemical Co. Ltd., China) was used as a growth medium. Bacterial suspensions were prepared by growing the bacteria overnight at 28 °C in TSB. Bacterial cells were harvested by centrifugation (4000 r/min, 15 min, 4 °C), washed twice with phosphate buffer solution (PBS (137 mmol/L NaCl, 2.7 mmol/L KCl, 7.0 mmol/L Na2HPO4·2H2O, 1.5 mmol/L KH2PO4)) and suspended at a density of 1 × 106– 2 × 106 cells/mL; then they were used for the antibacterial assays.
Based on National Committee for Clinical Laboratory Standards (NCCLS), antibacterial activity of Ag-CPC was evaluated by determination of minimum inhibitory concentration (MIC). The lowest antimicrobial concentration which inhibited bacterial growth after incubation for 24 h at 37 °C was considered the MIC [46] and [47]. Ag-CPC and CPC-0 were diluted in TSB gradually; and the bacteria was added to achieve a bacterial concentration of 1 × 106– 2 × 106cells/mL.
The as-processed Ag-ACP was analyzed using XRD (X‘Pert Pro, PANalytical, Netherlands) (Co). The content of silver in Ag-CPC was observed with Atomic Absorption Spectrometry (Z-5000, Hitachi, Japan). The surface chemical composition of dried Ag-CPC was tested by XPS (Axis Ultra DLD, Kratos, Britain). A standard Al K α excitation source (15 kV, 10 mA) was employed. Microstructure of Ag-CPC was observed by SEM (H-800, Hitachi, Japan) on gold-coated sample at a magnification of 5000.
Silver ion releasing from Ag-CPC into Trypticase (Tryptic) Soy Broth (TSB) was examined. At each time point (2, 6, 12 and 24 h), solution (1 mL) was taken out and centrifuged at 8000 r/min for 15 min at 28 °C; and the supernatant was collected. The concent of silver ion in supernatant was measured by atomic absorption technique (Z-5000, Hitachi, Japan).
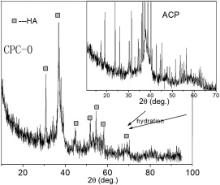
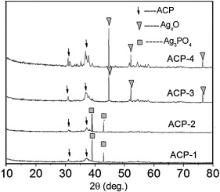
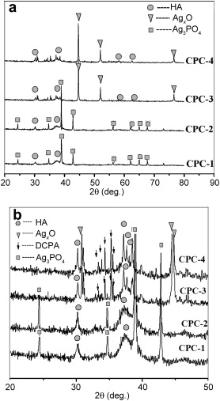
Fig. 1 shows XRD patterns of ACP without Ag and its hydration product (CPC-0). ACP shows the amorphous crystallized phase, which is different from a-TCP, β-TCP and TTCP. In the hydration reaction of CPC, ACP and DCPA reacted to form the poorly crystallized hydroxyapatite (HA) (JCPDS 09-0432), and the hydration product has the similar XRD diffraction to natural bone mineral. XRD patterns of Ag-ACP are shown in Fig. 2. It can be seen from Fig. 2 that ACP-1 and ACP-2 are in poor crystalline phase. After heat treatment at 460 °C, XRD diffraction peak of ACP-1 and ACP-2 (named as ACP-3 and ACP-4) is intensified. The XRD patterns of silver in ACP-1 and ACP-2 are in line with that of Ag3PO4(JCPDS 00-001-1058) ( Fig. 2). After heat treatment of 460 °C, the XRD patterns of silver in ACP-3 and ACP-4 are changed; but XRD patterns of silver are different from the usual silver-containing substances, such as Ag (JCPDS 00-001-1167), Ag2O (JCPDS 00-001-1041) and AgO (JCPDS 00-022-0472). This silver-containing substance in Fig. 2is named as Ag xO temporarily. Fig. 3 shows the XRD patterns of Ag-CPC. After setting, poorly crystallized HA are the major hydration products for all CPC groups, and the XRD patterns of silver are similar to that of Ag-ACP.
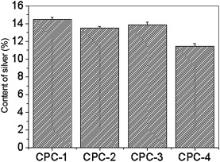
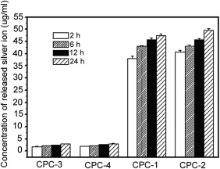
The contents of silver in Ag-CPC are measured by atomic absorption spectrometry, and the results are shown in Fig. 4. The contents of silver in all the CPC groups are more than 10%. Silver-releasing behavior is tested by atomic absorption technique. The contents of silver ion released from CPC groups are shown in Fig. 5. As time passes, the contents of released silver ion from all the CPC groups are trending up; the amount of silver ion released from CPC-1 and CPC-2 is much more than that from CPC-3 and CPC-4. Combined with the results shown in Fig. 4, we can conclude that different Ag-CPC groups exhibit different silver ion releasing behaviors, which is closely related to the valence of silver in CPC.
The antibacterial properties of Ag-CPCs are characterized by MIC assay, and the results are shown in Table 2. We can reach a conclusion that all Ag-CPC groups exhibit better antibacterial activity than the CPC without silver; and CPC-1 and CPC-2 show better antibacterial activity than CPC-3 and CPC-4.
| Table 2. MIC of Ag-CPC and CPC-0 (mg/L) |
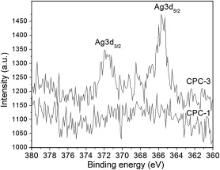
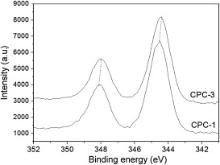
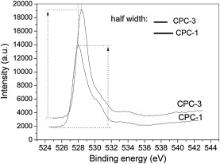
With the purpose of identifying the valence of silver in CPC, XPS is used to characterize the fracture surface of Ag-CPC. CPC-1 and CPC-3 are chosen as XPS samples. XPS spectra of silver (Ag 3 d states) deposited on the fracture surface of CPC-1 and CPC-3 is illustrated in Fig. 6. CPC-1 exhibits relatively weak XPS peak in Fig. 6; but after heat treatment at 460 °C, the Ag XPS in CPC-3 shows a typical double peak of high valence silver. According to XRD analysis, the XRD peaks of silver in CPC-1 are obvious ( Fig. 3 and Fig. 4); but in the XPS results ( Fig. 7), the XPS peaks of silver in CPC-1 are relatively weak. Fig. 8 represents the O 1s XPS spectra of CPC-1 and CPC-3. The O 1s peaks of CPC-1 and CPC-3 are 528.2 eV and 528.6 eV, respectively, which are less than that of AgO (528.8 eV) and Ag2O (529.2 eV) [47]. In addition, the peak height and full width at half maximum (FWHM) of O 1s of CPC-1 are different from that of CPC-3.
Fig. 9 illustrates the SEM images of Ag-CPC. The hydration morphology in cement can demonstrate how silver affects the hydration reaction of CPC. With the silver doping, hydration morphology of CPC groups changes obviously. The SEM graph of CPC-0 shows the hydration morphology of cement without Ag doping, and the crystal and amorphous phase intertwines during the growth process. With Ag doping, the hydration crystals become smaller (CPC-1) and cover on the surface of CPC (CPC-3).
In our research, silver is substituted into ACP from an aqueous solution of Ca(NO3)2·4 H2O, AgNO3and (NH4)2HPO4·12H2O by chemical precipitation method. It was reported that increasing the reactive temperature could boost the dehydration of hydrated Ag ion [48]. The different reaction temperature (25 °C and 60 °C) and heat treatment (460 °C) are used to adjust the different valence of silver in as-prepared CPC. It is concluded from Fig. 2that the silver of ACP-1 and ACP-2 shows similar XRD patterns to that of Ag3PO4(JCPDS 00-001-1058). XRD patterns of silver in ACP are changed under the heat treatment at 460 °C (ACP-3 and ACP-4). From the XRD analysis, it is concluded that low synthesis temperatures (25 °C and 60 °C) are not likely to change valence of silver in ACP, and Ag3+is the major valence (ACP-1 and ACP-2); while heat treatment can significantly change valence of silver (ACP-3 and ACP-4). During the hydration reaction of Ag-CPC, Ag-ACP reacts with DCPA to form poorly crystallized HA with silver doping. According to XRD analysis, there is no difference for valence of silver between Ag-ACP and Ag-CPC ( Fig. 3). Based on previously mentioned results, it is concluded that heat treatment on Ag-ACP changes silver's valence; but hydration reaction of Ag-CPC has no effect on the silver's valence.
Combined with XPS analysis, silvers in ACP-1 and ACP-3 exhibit different bonding energy ( Fig. 6). XPS patterns of Ag in CPC-3 show typical double peaks of high valence silver, and the value is different from that of Ag, Ag2O, and AgO (368.2, 367.8, and 367.4 eV, respectively) [49]. Hydration reaction of Ag-CPC is likely to affect XPS results. The XRD patterns of silver in Ag-CPC are not consistent with the usual silver-containing substances, such as Ag, Ag2O and AgO. It is possible that a new phase of silver is formed.
Fig. 5 and Fig. 6 demonstrate that silver in CPC-1 and CPC-2 exhibits better releasing property. After heat treatment at 460 °C, the amount of silver ion released from CPC decreases significantly (CPC-3 and CPC-4 in Fig. 5). Based on results of XRD and XPS analysis, silver ion in CPC-1 and CPC-2 exists in Ag3PO4; and XPS results indicate that the silver compounds in CPC-1 and CPC-2 have weak XPS peaks. After heat treatment at 460 °C, silver is more stable in CPC-3 and CPC-4. Therefore, it is relatively easy for silver ion to release from CPC-1 and CPC-2 compared with CPC-3 and CPC-4. The hydration reaction of Ag-CPC is a solution-precipitation process, during which other element (Ag) will be doped to form poorly crystallized HA as the hydration product [50]. Silver substitution decreases the bonding energy of calcium and increases the bonding energy of oxygen in CPC ( Fig. 7 and Fig. 8). The change of bonding energy of Ca and O may be resulted from Ag substitution in HA under heat treatment. Bonding energy of element is correlated with the electronegative value of an element in compound. Therefore, O 1s peaks of CPC-1 and CPC-3, including the peak height and FWHM, change significantly in different chemical environments ( Fig. 8). All of these factors induce the change of releasing behavior of silver from Ag-CPC ( Fig. 5). The releasing behavior of Ca/P is directly correlated with HA crystallinity, which will affect the releasing behavior of silver in HA. In our work, the releasing behavior of silver from CPC was tested during 24 h ( Fig. 5). In such a short term, we think the valences of silver were the major reason for different releasing properties. The releasing property of silver ion from CPC is the most important factor for its antibacterial capability. Besides, the antibacterial ability of Ag-CPC can be adjusted by changing the valence of silver in Ag-CPC.
Several proposals have been developed to explain the inhibitory effects of Ag ion on bacteria [22], [51] and [52]. However, the mechanism of antimicrobial effects of silver is still not clear. The antimicrobial effects of CPC doped with silver indeed have a close relationship with the releasing behavior and valence of silver in CPC. Besides, different Ag-CPC groups demonstrate different hydration morphology ( Fig. 9), which is closely related with silver doping or synthesis process. According to releasing tests, it is concluded that different valences of silver have different releasing property and antibacterial effect ( Fig. 5 and Table 2); and all the Ag-CPCs have better antibacterial effect than CPC without silver.
In this work, silver in CPC exhibited different bonding energy at various temperatures, which released different amounts of silver ion correspondingly. All samples showed stable silver release, and CPC-1 and CPC-2 demonstrated a high silver-releasing ability. The antimicrobial activity of CPC was tested by E. coli ; the results indicated that different valence of silver exhibited different antibacterial activity, which was in line with the silver-releasing results. This study may provide a new route in the design of antibacterial CPC for efficient bone tissue regeneration.
This work was supported by the National Natural Science Foundation of China (No. 51172074; No. 51302089); the Natural Science Foundation of Guangdong Province of China (No. 04205786); the Hong Kong Scholars Program (No. XJ2011010); China Postdoctoral Science Foundation (CPSF, No. 2012M511571, 201104358); Guangdong Natural Science Foundation (No. S2012040007845); the Fundamental Research Funds for the Central Universities, SCUT (No. 2013ZZ0010).
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|
| 18. |
|
| 19. |
|
| 20. |
|
| 21. |
|
| 22. |
|
| 23. |
|
| 24. |
|
| 25. |
|
| 26. |
|
| 27. |
|
| 28. |
|
| 29. |
|
| 30. |
|
| 31. |
|
| 32. |
|
| 33. |
|
| 34. |
|
| 35. |
|
| 36. |
|
| 37. |
|
| 38. |
|
| 39. |
|
| 40. |
|
| 41. |
|
| 42. |
|
| 43. |
|
| 44. |
|
| 45. |
|
| 46. |
|
| 47. |
|
| 48. |
|
| 49. |
|
| 50. |
|
| 51. |
|
| 52. |
|