In fabricating magnesium-matrix composites, an easy and cost-effective route is to infiltrate the ceramic preform with molten Mg without any external pressure. However, a rather well wettability of molten Mg with ceramic reinforcement is needed for this process. In order to improve the wettability of the metal melt with ceramic preform during fabricating composites by metal melt infiltration, a simple and viable method has been proposed in this paper where a small amount of metal powder with higher melting point is added to the ceramic preform such that the surface tension of the Mg melt and the liquid–solid interfacial tension could be reduced. By using this method, boron carbide particulate-reinforced magnesium-matrix composites (B4C/Mg) have been successfully fabricated where Ti powder immiscible with magnesium melt was introduced into B4C preform as infiltration inducer. The infiltration ability of molten Mg to the ceramic preform was further studied in association with the processing conditions and the mechanism involved in this process was also analyzed.
Light-weight particulate-reinforced magnesium-matrix composites are gradually finding wide applications as promising structural and functional materials in electronic, aeronautical and aerospace fields, since they possess high specific strength and stiffness, excellent wear-resistant ability, good electrical and thermal conductivities, and vibration-damping capacity [1], [2], [3] and [4].
As for the fabrication of magnesium-matrix composites, several routes have been utilized to synthesize magnesium-matrix composites, which mainly include powder metallurgy [5], stir casting [6] and infiltration technique with or without the aid of pressure [7] and [8]. Recently, Habibi et al. [9] utilized powder metallurgy route using microwave assisted rapid sintering technique followed by hot extrusion to synthesize magnesium nano-composites in order to enhance the strength and ductility of magnesium simultaneously. Interpenetrating metal-matrix composites were prepared by Scherm et al. [10] through infiltrating of porous ceramic performs by a pressure supported casting process and the microstructure was also characterized by using different experimental methods. Composites containing pure magnesium and hybrid reinforcements were synthesized by Sankaranarayanan et al. [11] through disintegrated melt deposition technique (DMD) followed by hot extrusion and the effect of ball milling the hybrid reinforcements on the microstructure and mechanical properties of this kind of composites was also evaluated. Among these routes, metal melt infiltration into ceramic preform is recognized as having merits in low cost, high efficiency, near net-shape, availability of composites with high ceramic volume fraction and received much attention in recent years [12] and [13]. However, the prerequisite for effective infiltration of metal melt into the ceramic preform is good wettability of metal melt to the ceramic particulate [2]. Generally, the wettability between ceramic and magnesium melt is not so well and the infiltration process is unlikely to occur unless the fabrication temperature is extremely high, e.g. in conventional ceramic/magnesium systems as Mg–Ti–C, Mg–Ti–B, Mg–B4C. Thus, promotion of the infiltration ability becomes a critical issue in fabricating magnesium-matrix composites by using this technique.
The usual way to enhance the wettability of metal melt to the ceramics is to raise the temperature much higher than the melting point. Undoubtedly, this method is suitable for the non-volatile molten metal and can not be used for volatile metal, e.g. Mg and Zn. In this paper, an effective way to improve the wettability of the metal melt with ceramic preform was proposed in fabricating magnesium-matrix composites via pressureless infiltration technique by adding small amount of metal particles with high melting point.
As an illustrative case, this conception was demonstrated in fabricating B4C/Mg composite by magnesium melt infiltration into B4C ceramic preform where some amount of Ti particles had been added, and the mechanism of effective infiltration involved in this process was also analyzed. Since B4C has a lower density (2.52 g/cm3), even when the B4C takes 50% volume fraction, the composite system B4C/Mg is extra-light and the density is only about 2 g/cm3, much lighter than that of pure aluminum, a little higher than that of magnesium. It is worth noting that such a high volume fraction of ceramics in this composite system will bring about excellent wear resistance [14], so it will find wide applications in aeronautical and aerospace industries.
The raw materials used in fabricating boron carbide reinforced magnesium composites were listed in Table 1 where the B4C particulate has mean sizes of 28, 10 and 5 μm, respectively, and Ti particulate has an average size of 25 μm. The magnesium ingot was used as infiltration metal and it has a commercial purity of 99.95%.
| Table 1. Raw materials used in fabricating B4C/Mg composites |
There are two main steps in fabricating B4C/Mg composites, i.e. preparation of the B4C preform and spontaneous infiltration of magnesium melt into B4C ceramic preform. Firstly, Ti powder of different volume fraction (0%, 2%, 4%, 6% and 8%) was added into B4C powder. After adding a certain amount of binder and fully mechanical blending, the mixed Ti and B4C powders were then cold compacted into green body of cylindrical shapes (16 mm in diameter, height variable) in a steel mould with various relative densities of 50%, 55% and 60%. The compacted preform together with a pure magnesium ingot on it was put into a graphite mould. Several small holes were drilled at the bottom of the graphite mould in order to release the air during fabricating the B4C/Mg composites. The infiltration process was carried out in an electric furnace under the presence of a flowing argon (99.999% purity) atmosphere. The chamber of the furnace was degassed prior to heating and then backfilled with Ar. The infiltration system was finally heated up to temperatures of 953, 973 and 993 K, respectively, at a rate of 10 K/min. At each temperature, the holding time was all set as 90, 120 and 150 min. After that, the samples were cooled with the furnace down to the room temperature.
Metallographic microscopy (LEICA Q550IW), scanning electron microscopy (SEM, FEI Quanta 600) and X-ray diffractometer (XRD, X'Pert Pro) were used to characterize the microstructures and the phases of the as-infiltrated composites. The specimens were sectioned and polished in the infiltration direction in order to measure the infiltration distance to analyze the influence of processing parameters on the infiltration dynamics.
As expected, with the aid of Ti particulate in B4C preform the magnesium melt can spontaneously infiltrate into the ceramic preform of B4C when the heating temperature is at 953, 973, and 993 K for different holding time under the flowing argon atmosphere. It is noticeable that the infiltration process of magnesium melt into B4C preform does not occur without the addition of Ti particulate into B4C preform. This implies that the Ti particulate plays a predominant role in this process. That is to say, the B4C/Mg composites can be successfully fabricated with the existence of Ti as an infiltration inducer.
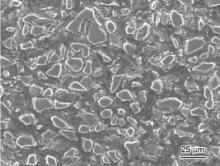
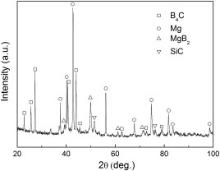
Fig. 1 shows the typical SEM micrograph of the as-prepared B4C/Mg composites at 973 K for 120 min. The original preform has a 4% volume fraction of Ti particles and a relative density of 60%. It can be seen in Fig. 1 that B4C particles with an irregular shape were uniformly distributed in the magnesium-matrix and very few Ti particles were found due to its extremely low volume fraction. Fig. 2 shows the XRD spectrum of the as-fabricated B4C/Mg composites corresponding to Fig. 1 and it contains four phases, i.e. B4C, Mg, MgB2and SiC. The presence of Mg and B4C phases confirms that magnesium melt has infiltrated into the B4C preform under the present processing conditions. No Ti peak was detected owing to its low addition and SiC was introduced as impurities during the blending of starting powders or the polishing of B4C/Mg composites samples using silicon carbide paper. Besides, there is a weak peak corresponding to MgB2trace phase. It was reported that B4C particles were easily oxidated during mixing the starting materials and an amorphous B2O3surface layer was produced. After that, an interfacial reaction of B2O3with magnesium melt occurred and rod-like MgB2produced [15].
It is obvious that the processing parameters, e.g. Ti content, heating temperature, holding time and relative density, have much influence on the fabricating process and thus the materials performance. For these reasons, a further study was carried out in order to have a detailed understanding of the infiltration process under the presence of Ti particles as infiltration inducer.
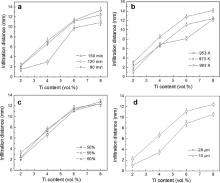
In order to study the infiltration ability or infiltration distance of magnesium melt in the ceramic preform, several processing parameters were taken into account in analyzing the infiltration process. The experimental results were collected in Fig. 3 showing the infiltration distance versus various processing parameters for preparing the composites. For the infiltration of magnesium melt into B4C preform, the infiltration process is unlikely to occur when no Ti was added into B4C particles, even at a rather high temperature or for extended time. Moreover, despite of all the other factors, the more the amount of Ti addition is, the greater the infiltration distance is. In Fig. 3(a), one can see that the infiltration distance increases with increasing the holding time, but the discrepancy of the infiltration distance at 120 min and 150 min is not evident, which means that extending holding time is not needed for fabricating B4C/Mg composites in view of efficiency. While in Fig. 3(b) the infiltration distance also increases when the processing temperature is raised. Fig. 3(c) demonstrates that a preform with higher relative density can lead to a larger infiltration distance.
It is well known that the particulate size of the raw powder materials is of great importance in the preparation of particulate-reinforced metal-matrix composites by spontaneously pressureless infiltration technique. In this study, three kinds of particle size, 5 μm, 10 μm and 28 μm, of B4C powder were used to study their influence on the infiltration process. When the B4C powder with particle size of 5 μm was used, the process of magnesium melt infiltration into B4C ceramic powder did not take place. It is obvious in Fig. 3(d) that a larger infiltration distance can be obtained with larger B4C particle size. In other words, when the particle size of B4C powder is comparable to that of Ti particles, the infiltration process is prone to occur.
For Mg–B4C system, the infiltration of magnesium melt into B4C preform depends largely on the wettability between them. The capillarity acts as the driving force without any external applied pressure. The wettability of a liquid with a solid is generally measured by the contact angle θ. And the angle θ is related to the surface tension γl/gof the interface of liquid and gas, which can be expressed by the Young–Kelvin equation [16]:
where p is the capillary force and r the radius of the pores within the preform. Eq. (1) indicates that a liquid can wet a solid only when the contact angle θ is less than 90°, i.e. cos θ is positive. In more detail, when the contact angle is smaller and the liquid–gas surface tension is greater, or the pore radius is smaller, the penetration is easier to occur.
A schematic representation of the spontaneous infiltration process is illustrated in Fig. 4 with the existence of Ti particles as infiltration inducer. It can be seen that the magnesium melt penetrated into the ceramic preform along the pores between Ti and B4C particles by the capillarity. Once the magnesium melt come across Ti particulate, the infiltration of magnesium melt into the B4C preform is accelerated. The B4C/Mg composites were finally fabricated after the magnesium melt infiltrated into the full B4C ceramic preform.
 | Fig. 4. Schematic illustrations showing the infiltration of magnesium melt into a porous preform containing Ti particulates by capillarity in fabricating B4C/Mg composites. |
Generally, the wettability of magnesium melt with B4C is not so well that the penetration proceeds at an extremely low rate. This can be observed in the experiment without Ti added to the B4C particles. Some research indicates that a suitable third metal can be added to decrease the surface tension of the melt and the liquid–solid interfacial tension, thus to improve the wettability [17] and [18]. As described in Ref. [19], a sessile drop technique was utilized to study the wetting behavior of copper alloys on SiC substrates and the results showed that Ti and Cr are effective elements to improve the wettability owing to lowering the interfacial energy or promoting the chemical reaction. Moreover, the metal to be used mostly tends to be chemically active. For example, minor magnesium in aluminum matrix composites plays an important role in improving the wettability of the aluminum melt and reinforcement through aiding the reaction at the surface of the reinforcement particles and forming new compounds at the interface [20]. In this study, however, the infiltration mechanism differs from that. Ti has higher melting point than magnesium and is immiscible with magnesium. When it was added, the surface tension of the melt or liquid–solid interfacial tension would be reduced. Thus, the wettability of the melt and preform can be improved and it could lead to the penetration of the melt into ceramic preform at the same time.
In order to obtain a more uniformly distributed B4C particulate-reinforced magnesium-matrix composites, numerous infiltration experiments have been conducted to have an optimal processing condition. As an illustrative case, Fig. 5 shows the optical micrographs of the as-fabricated B4C/Mg composites with different amount of Ti under the condition at 993 K for 120 min where the relative density of the preform is 50%. It can be seen that homogeneous distribution of the ceramic reinforcement B4C in the microstructure could be obtained when the volume fraction of Ti was 8%. In fact, when the volume fraction of Ti reaches 6% or above, B4C/Mg composites could be fabricated with uniform microstructures and more infiltration distance, which can be seen in Fig. 3. For the composites B4C/Mg containing 8 vol.% addition of Ti powder, the density was measured to be 2.147 g/cm3according to Archimedes's principle, which is much lighter than aluminum. The hardness of the as-fabricated B4C/Mg composites was also measured in the range of 110 ± 2 HB by using Full-Automatic Rockwell Type Hardness Tester, about 3–3.6 times higher than that of pure magnesium.
(1) By introducing a small amount of metal powder Ti with higher melting point than Mg to the B4C ceramic preform as infiltration inducer, B4C/Mg composites could be easily and cost-effectively prepared by Mg melt infiltration technique. The mechanism involved in this process is that the wettability of magnesium melt to B4C ceramic preform is largely improved by reducing the surface tension of the melt and the liquid–solid interfacial tension.(2)
(2) In fabricating B4C/Mg composites by metal-assisted melt infiltration technique, there are several factors that have influences on the process, including fabricating or heating temperature, holding time, relative density of the ceramic preform and particle sizes of the starting powders. Generally, these factors will play positive roles in melt infiltration ability, i.e. with increasing the heating temperature, holding time or the relative density of the ceramic preform, the infiltration distance is increased. Moreover, when the size of Ti particulates is comparable to that of B4C particles, it would be beneficial to the spontaneous infiltration process.(3)
(3) In order to obtain B4C/Mg composites with homogeneously distributed B4C particles within the magnesium-matrix, it is required that 6 vol.% or above of metal powder Ti is added into B4C ceramic preform. For the B4C preform with relative density of 50%–60%, a processing condition at 993 K for 120 min is suitable for fabricating a dense B4C/Mg composites.
Financial support from the National Natural Science Foundation of China (Grant No. 51271051) is greatly appreciated. The authors thank Senior Engineers Yinxuan Gao, Zhenwei Zhang, and Mrs Yanhua Zhang, Institute of Metal Research, Chinese Academy of Sciences, for their support in the infiltration experiment.
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|
| 18. |
|
| 19. |
|
| 20. |
|