A new three-component and magnetically responsive NiFe2O4@PANI@Ag nanocomposite has been fabricated by coating of nickel ferrite, NiFe2O4, nanoparticles with polyaniline (PANI) and subsequent immobilization of silver nanoparticles onto the surface of polyaniline shell. The as-prepared nanocomposite has been characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), and vibrating sample magnetometer (VSM). The saturation magnetization of the NiFe2O4 core decreases dramatically after coating with polyaniline and silver nanoparticles, however, the nanocomposite NiFe2O4@PANI@Ag can be still separated from solution media through magnetic decantation. The antibacterial activity of the synthesized nanocomposite was studied and compared with those of naked NiFe2O4, NiFe2O4@PANI and some standard antibacterial drugs.
Composite materials with core/shell structure have attracted the attention of the scientific community because of their potential for combining properties that are difficult to attain separately with the individual components [1] and [2]. Core/shell materials comprising a ferromagnetic component and a conducting polymer have recently been investigated in several studies [3] and [4]. Among the various intrinsically conducting polymers, polyaniline has emerged as the most promising conducting polymer due to its unique electrical, optical properties, easy polymerization, high environmental stability and low cost of monomer [5], [6] and [7]. Therefore, one of the most common methods for synthesizing magnetic core/shell nanocomposite materials was coating of spinel ferrites, such as Fe3O4, CoFe2O4, NiFe2O4, MnFe2O4, and ZnFe2O4, with polyaniline (PANI). Coating is usually achieved by in situ polymerization of aniline in the presence of magnetic ferrite nanoparticles [8], [9] and [10]. When a spinel ferrite is coated with polyaniline, a magnetic electrical conducting composite is prepared. The electrical conductivity of these two-component composites varie with the thickness of the polyaniline shell [11], [12] and [13].
To date, several PANI/metal oxides two-component nanocomposites have been synthesized and their properties have been studied [1], [14], [15] and [16]. However, three-component composites resulted from combination of the magnetic core/shell particles together with other functional materials, such as noble metals, are limited in number and have not yet been widely explored. Xuan et al. [17]have synthesized Fe3O4@PANI@Au nanocomposite via coating of Fe3O4with polyaniline and subsequent assembling of Au nanoparticles onto the surface of polyaniline shell. Babayan et al. [18]have also reported the synthesis of a hybrid composite consisting of MnZn ferrite, polyaniline, and silver particles. This latter composite was obtained by oxidative polymerization of aniline with silver nitrate in the presence of ferrite powder. Interestingly, there was no need for the use of an acid and oxidant for the polymerization of aniline in the reported method. To the best of our knowledge, there are no other reports covering the synthesis of these types of three-component core/shell/shell nanocomposites, containing polyaniline as first shell and noble metal nanoparticles as second shell, besides the above mentioned two examples. However, there are many reports in the literature discussing the synthesis and the properties of other three-component composites, in which the polymer shell is not polyaniline [19], [20] and [21].
Accordingly, we have successfully synthesized a new magnetically responsive NiFe2O4@PANI@Ag nanocomposite through a straightforward and facile three-step procedure. The magnetic core, NiFe2O4, was synthesized first by a combustion method and coating of this core with polyaniline was carried out by in situ chemical oxidative polymerization of aniline. The as-prepared NiFe2O4@PANI core/shell composite was then decorated with silver nanoparticles via a chemical reduction of silver nitrate in an aqueous solution. The NiFe2O4@PANI@Ag nanocomposite was characterized by various techniques and its antibacterial activity was also studied. This composite can be readily isolated from solution media by applying an external magnetic field. Therefore, NiFe2O4@PANI@Ag composite is considered as a superior antibacterial agent compared to bare silver nanoparticles, since the Ag based disinfectants are toxic and must be removed from water after treatment [22].
All the chemicals, including nickel (II) nitrate, iron (III) nitrate, silver nitrate, ammonium peroxydisulfate, and polyvinylpyrrolidone were provided from Merck and used without further purification. Double distilled, deionized water was used as a solvent in all reactions. Manipulations and reactions were carried out in air without the protection of nitrogen or other inert gas. Fourier transform infrared (FT-IR) spectra were obtained using an FT BOMEM MB102 spectrophotometer. X-ray diffraction (XRD) patterns of the synthesized samples were taken with a Philips X-ray diffractometer (Model PW1840) over a 2 θ range from 10° to 90° using Cu K α radiation ( λ = 0.154056 nm). The scanning electron microscopy (SEM) images were obtained using a Hitachi Japan S4160 scanning electron microscope. Magnetic properties of the as-prepared composites were studied using vibrating sample magnetometer (VSM) from Meghnatis Daghigh Kavir Company.
Nickel ferrite nanoparticles were prepared by a combustion method using nickel (II) and iron (III) nitrates with known amount of glycine, as a fuel, with molar ratio of 1:2:6 [23] and [24]. The mixed precursors were concentrated in a porcelain crucible on a hot plate at 300 °C. After the mixture reached the point of spontaneous combustion, it began to burn and release lots of gases, vaporize all the liquid instantly yielding a black voluminous and fluffy NiFe2O4product in the container. The product was washed with water and ethanol and dried in an oven at 80 °C for 3 h.
The PANI@NiFe2O4composite was prepared by precipitating of PANI on the surface of NiFe2O4nanoparticles. In this procedure, 50 mL of freshly prepared reaction mixture (0.1 mol/L aniline, 0.125 mol/L ammonium peroxydisulfate in 0.5 mol/L nitric acid) was added to 1.0 g of nickel ferrite at 20 °C. The mixture was stirred during the polymerization of aniline, which was completed within 2 h. The polymerization process was identified by the change of the starting colorless solution into deep green color. The ferrite-PANI composite was separated magnetically, washed with double distilled water and methanol for several times. Finally, the collected sample was dried in a vacuum oven at 60 °C for 6 h.
A reduction chemical method was employed for the immobilization of Ag nanoparticles onto the surface of NiFe2O4@PANI composite to prepare the three-component NiFe2O4@PANI@Ag nanocomposite. In the first step, 1.0 g of NiFe2O4@PANI was dispersed in AgNO3aqueous solution (0.156 mol/L). After vigorous stirring of this mixture for 30 min to ensure the adsorption of Ag+ions by the composite nanoparticles, sodium hydroxide solution with a fixed quantity of polyvinylpyrrolidone (PVP), as stabilizer polymer, was added. Finally, a solution of glucose (C6H12O6), as reducing agent, was introduced into the reaction vessel and the whole mixture was heated at 70 °C to accelerate the reduction reaction. After fixed period of reaction time, the prepared NiFe2O4@PANI@Ag composite was separated by magnetic decantation and washed several times with water to remove any excess protecting agent and alkaline materials. The solid was dried at 80 °C for 5 h. The silver content of the NiFe2O4@PANI@Ag nanocomposite was determined by atomic absorption spectroscopy (AAS) analysis. The composite was first dissolved in concentrated nitric acid and the AAS analysis was carried out to measure the amount of silver in this solution. The silver content of this composite was found to be 12% ( w / w).
Antibacterial susceptibility tests were carried out on the as-prepared magnetic materials, based on Kirby–Bauer disk diffusion method. In these tests, three concentrations including 10, 20 and 40 mg/mL of NiFe2O4, NiFe2O4@PANI and NiFe2O4@PANI@Ag were prepared in sterile water and dispersed by sonication. Blank disks with 6.4 mm (in diameter) were saturated by adding 30 μl of each of the prepared magnetic nanoparticle suspension and then placed on Mueller-Hinton agar culture medium (Merck, Germany) inoculated with standard bacterial species. The tested bacteria were of Gram-Positive and Gram-Negative including, Staphylococcus aureus , Bacillus subtilis , Escherichia coli and Pseudomonas aeruginosa. A lawn culture with 0.5 McFarland turbidity was prepared using sterile cotton swabs on culture medium. The plates were incubated at 37 °C for 24 h and then the inhibition zone around each disk was measured and recorded based on mm. The results of this study are discussed in the following section.
The oxidation of freshly distilled aniline in an acidic aqueous medium by ammonium peroxydisulfate yields PANI as green powder [25] and [26]. When the oxidative polymerization of aniline is carried out in the presence of nickel ferrite nanoparticles, the two-component composite, NiFe2O4@PANI, is easily formed. The surface of the as-prepared NiFe2O4@PANI nanocomposite was then inlaid with silver nanoparticles via a chemical reduction method [27]. In this procedure, silver nanoparticles were prepared by reducing silver nitrate in polyvinylpyrrolidone (PVP) aqueous solution. Glucose was used as reducer agent for Ag+ions and sodium hydroxide for accelerating this reduction reaction (see).
In this procedure nitric acid solution has been used as a medium for the in situ polymerization of aniline because nitrate counter-ions in the final PANI coating have no interaction with the Ag+ions during the immobilization of Ag nanoparticles onto the NiFe2O4@PANI composite. Other acids, such as hydrochloric or phosphoric, are not suitable because silver nitrate will precipitate as silver chloride or silver phosphate during the reaction.
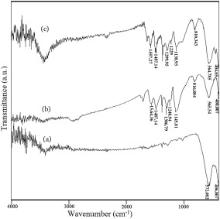
Polymerization of aniline was confirmed by the FT-IR spectra in the range of 4000–400 cm-1 at ambient temperature. The FT-IR spectra of NiFe2O4, NiFe2O4@PANI, and NiFe2O4@PANI@Ag nanoparticles are shown in Fig. 1. In all these spectra two main absorption bands at around 570 and 400 cm-1 are observed. These bands are attributed to the intrinsic stretching vibrations of iron ions in tetrahedral and octahedral sites of the spinel structure, respectively. However, as seen in Fig. 1, the FT-IR spectra of NiFe2O4@PANI and NiFe2O4@PANI@Ag nanocomposites, exhibit all the characteristic peaks of polyaniline in addition to the Fe–O stretching vibrations [28] and [29]. In the spectrum of NiFe2O4@PANI@ composite, the bands position at 1564.36 cm-1 and 1487.14 cm-1 were attributed to the characteristic C–C stretching of the quinoid and benzenoid rings while the bands observed at 1306.79 cm-1 was assigned to C–N stretching of the secondary aromatic amine. The bands at position 1145.81 and 816.88 cm-1reflected the aromatic C–H in-plane bending and out-of-plane deformation of C–H in the 1, 4-disubstituted benzene ring, respectively [15]. More or less the same peaks were observed in the FT-IR spectrum of NiFe2O4@PANI@Ag nanocomposite (see Fig. 1(c)). This observation clearly indicates that the NiFe2O4magnetic core was coated with polyaniline shell to form a core/shell nanostructure in both NiFe2O4@PANI and NiFe2O4@PANI@Ag nanoparticles.
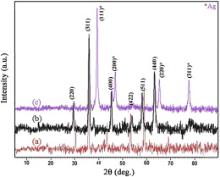
The crystallinity and structure of the prepared nanocrystals were confirmed by XRD. The XRD patterns of NiFe2O4, NiFe2O4@PANI and NiFe2O4@PANI@Ag samples are presented in Fig. 2. In the pattern of NiFe2O4, six sharp diffraction peaks are observed, which correspond to ( hkl) planes of (220), (311), (400), (422), (511) and (440), respectively. All of the detectable peaks could be identified as characteristic diffraction of spinel NiFe2O4 standard data, in JCPDS 44-1485. The diffractogram exhibits sharp lines indicating that the as-prepared NiFe2O4sample has high crystallinity.
From the XRD data, the crystallite size of the as-synthesized NiFe2O4 particles was calculated to be around 38 nm, using Debye–Scherrer equation [30]. The XRD pattern of NiFe2O4@PANI nanocomposite shows all the above mentioned characteristic peaks of NiFe2O4core. This reveals that the spinel structure of NiFe2O4magnetic core remains intact on coating with PANI. In the XRD pattern of NiFe2O4@PANI@Ag nanocomposite, however, four characteristic peaks for Ag were also found at 2 θ of 38.2°, 44.4°, 64.6° and 77.5°, marked by their indices (111), (200), (220), and (311). These are consistent with the database in JCPDS file (JCPDS No. 4-783), and indicated that Ag with face-centered cubic symmetry structure was included in this composite [31].
The morphology of the as-prepared nanomaterials was determined by SEM. From SEM images ( Fig. 3) it is observed that, uncoated NiFe2O4nanoparticles show highly agglomerated porous foam-like structure, which is characteristic of combustion synthesis [32]. The SEM micrographs of NiFe2O4@PANI and NiFe2O4@PANI@Ag composites, however, show rather well dispersed sphere-like nanostructures with few agglomerates. This may be due to the coating of PANI on the surface of NiFe2O4nanoparticles. The polymer is coated on the surface of NiFe2O4nanoparticles core due to in situ polymerization. As reported in literature [14] and [33], the interaction between PANI shell and the oxygen atoms of the ferrite core leads to embedment of ferrite particles on PANI chains. The particle sizes of the as-prepared NiFe2O4@PANI and NiFe2O4@PANI@Ag samples were about 60 and 70 nm, respectively.
The magnetic properties of all the prepared nanomaterials were investigated by VSM at room temperature. Fig. 4 displays the VSM magnetization curves of the bare NiFe2O4nanoparticles, NiFe2O4@PANI and NiFe2O4@PANI@Ag nanocomposites. The saturation magnetization ( Ms), coercivity ( Hc) and remnant magnetization ( Mr) of these materials were determined from the hysteresis loops measurements and are shown in Table 1. It was observed that the values Ms, Mr and Hcfor NiFe2O4@PANI nanocomposite ( Fig. 4(b)) are less than those obtained for pure NiFe2O4. Further decrease in the magnetic nature of the naked ferrite was noticed for the three-component NiFe2O4@PANI@Ag composite ( Fig. 4(c)). This behavior is due to the non-magnetic PANI and Ag coatings, which can be considered as magnetic dead layers on the magnetic core surface, thus affecting the magnitude of magnetization due to quenching of the surface moment [12] and [34]. In other words, according to the equation Ms= φms, where φ is the volume fraction of the magnetic particles and msis the saturation moment of a single particle, it is expected that Ms value of the composites is dependent on the volume fraction of the magnetic ferrite particles ( φ). Hence, due to the contribution of the non-magnetic PANI and silver coating layers to the total magnetization, the NiFe2O4@PANI and NiFe2O4@PANI@Ag nanocomposites have less magnetization than what observed for the uncoated ferrite nanoparticles. Interestingly, although the saturation magnetization of the NiFe2O4 core is significantly decreased on coating (from 73.35 to 15.4 emu/g), the NiFe2O4@PANI@Ag composite can be still efficiently isolated from solution media by applying an external magnetic field.
| Table 1. Magnetic parameters of the as-prepared magnetic materials |
The silver content of the NiFe2O4@PANI@Ag nanocomposite was also determined by AAS and was found to be 12% ( w / w). This value is in a close agreement with the silver content of the earlier reported Fe3O4–SiO2–Ag and MnZnFe2O4–PANI–Ag magnetic composites [18] and [35], however, much higher than the Ag content of Ag–Fe3O4nanocomposite [36].
Although silver has a potent antibacterial activity, the use of Ag nanoparticles as therapeutic agent is limited due to their potential cytotoxic activity against mammalian cells [22] and [37]. Therefore, when silver nanoparticles are used as disinfectant, they must be removed from water after disinfection because of their toxicity for the aqua system. The removal of silver nanoparticles from solution media is one of the problems that limit their application. Using the nanocomposite NiFe2O4@PANI@Ag, as disinfectant, instead of silver nanoparticles, however, may solve the encountered problems since this composite can be easily removed magnetically from the solution and possible contamination of disinfectant to environment is avoided. Therefore, it is worthwhile to investigate the antibacterial activity of NiFe2O4@PANI@Ag nanocomposite. As can be seen from the data of Table 2, the antibacterial activity of NiFe2O4@PANI@Ag nanocomposite is higher than that of NiFe2O4 and NiFe2O4@PANI magnetic nanomaterials and also higher than that of some of the standard antibacterial drugs. Moreover, immobilization of Ag nanoparticles onto the polyaniline matrix of the nanocomposite can exert their antimicrobial activity but it is expected that their cytotoxic effect on mammalian cells will be less than that of free silver nanoparticles because of slow release of silver ions from the polyaniline matrix.
| Table 2. Antibacterial activities of NiFe2O4, NiFe2O4@PANI and NiFe2O4@PANI@Ag |
Nickel ferrite nanoparticles have been synthesized by a facile, inexpensive, nontoxic and reproducible combustion method. This ferrite was then coated with polyaniline via in situ chemical oxidative polymerization of aniline monomers nanoparticles to obtain NiFe2O4@PANI core/shell composite. The surface of this nanocomposite was subsequently decorated with silver nanoparticles via a reduction reaction to afford a new three-component NiFe2O4@PANI@Ag nanocomposite. The latter nanocomposite was characterized by XRD, FT-IR and SEM techniques. The magnetic properties of all the prepared materials were measured using VSM at room temperature. The magnetic measurements indicated that the Ms and Mr and Hc values of NiFe2O4core decrease on coating with polyaniline and silver nanoparticles. The antibacterial activity of the magnetically responsive NiFe2O4@PANI@Ag nanocomposite was also investigated and was found to be higher than that of NiFe2O4and NiFe2O4@PANI and some of the standard antibacterial drugs. Interestingly, this nanocomposite can be readily removed from the reaction or disinfected media via magnetic decantation.
The authors acknowledge the support of this study provided by the Research Council of Shahid Chamran University, Ahvaz, Iran.
| 1. |
|
| 2. |
|
| 3. |
|
| 4. |
|
| 5. |
|
| 6. |
|
| 7. |
|
| 8. |
|
| 9. |
|
| 10. |
|
| 11. |
|
| 12. |
|
| 13. |
|
| 14. |
|
| 15. |
|
| 16. |
|
| 17. |
|
| 18. |
|
| 19. |
|
| 20. |
|
| 21. |
|
| 22. |
|
| 23. |
|
| 24. |
|
| 25. |
|
| 26. |
|
| 27. |
|
| 28. |
|
| 29. |
|
| 30. |
|
| 31. |
|
| 32. |
|
| 33. |
|
| 34. |
|
| 35. |
|
| 36. |
|
| 37. |
|