Corresponding authors:
Received: 2019-02-4
Revised: 2019-03-13
Accepted: 2019-05-22
Online: 2019-10-05
Copyright: 2019 Editorial board of Journal of Materials Science & Technology Copyright reserved, Editorial board of Journal of Materials Science & Technology
More
Abstract
Segregation and inclusion precipitation are the common behaviours of steel solidification, which are resulted from the redistribution and diffusion of the solute elements at the solid-liquid interface. The effect of the phase transition of high-sulfur free-cutting steel is quantified in the present work for the solute partition coefficient (ki), inclusion precipitation, and microsegregation by establishing a coupling model of microsegregation and inclusion precipitation, wherein the quantified dependencies of ki in terms of temperature, phase and carbon (C) content were applied. Results showed that the solidification temperature range and phase transition of high-sulfur steel that under different solidification paths and C contents were quite different, leading to differences in ki and eventually in microsegregation. kC, kP, and kS were mainly affected by phase composition and kSi was primarily by temperature, while kMn depended on both phase composition and temperature during solidification. Increasing the C content within the interval 0.07-0.48 wt%, the ‘proportion of the δ phase maintained temperature region during solidification’ (Pδ), kPave and kSave (kiave, the average value of the ki across the whole stages of solidification) decreased monotonically, whereas kCave increased linearly. The peritectic reaction impacted on the phase composition and ki, leading to the change in microsegregation. Such effect of the peritectic reaction was more significant at the last stage of solidification. When the Pδ was between 75% and 100% (corresponding to 0.07-0.16 wt% C), the solidification path resulted in a greater effect on the microsegregation of solutes C, P, and S because of the peritectic reaction. The microsegregation of solutes Mn and S were comprehensively influenced by kMn, kS and MnS precipitation as well. The studies would help reveal the solute redistribution at the solid-liquid interface, and improve the segregation of high-sulfur steel by controlling the solidification and precipitation in practice.
Keywords:
The free-cutting steels have been widely applied in the fields such as metallurgy, machinery and aerospace because of the excellent machinability [1]. In the matrix of free-cutting steel, the inclusions played an important role in the lubricant effect during machining process, resulting in the improvement on the cutting performance [2]. The sulfur-treated steel which contains no heavy metals is one kind of environment-friendly free-cutting steels, and it has been developed rapidly [3]. During the solidification process of high-sulfur free-cutting steel, the solute redistribution and the precipitation of MnS inclusion were quite different from that in the traditional steels because of the higher S content [4,5]. Therefore, studying on the characteristics of the microsegregation and MnS precipitation for high-sulfur steel solidification are of great significance.
Segregation is one of the natural properties for an alloy solidification. It is the phenomenon of the uneven distribution of solute elements in the solidification structure of an alloy because of the redistribution of solute elements between the solid and liquid phases during solidification [6,7]. In the continuous casting process of steel, segregation is difficult to be eliminated completely. Serious segregation would deteriorate the service life and performance of the steel products [[8], [9], [10]]. To improve the segregation and ensure the quality of products, a series of studies on the influencing factors and improvement measures for segregation were carried out by simulation and experimental methods [9,[11], [12], [13], [14]]. In the study of segregation, the microsegregation model, which fundamentally determined the redistribution trend of solute elements at the solidification front, was the theoretical basis [15]. Therefore, to predict the segregation more accurately, it is necessary and has a realistic meaning to develop a comprehensive model.
Over the past decades, many foundation microsegregation models were proposed based on various assumptions. The Lever model and Scheil model, which assumed the complete diffusion of solute element in the liquid and/or not in the solid phases, were two of the most simplest models [15,16]. In order to display the diffusion of solute element in the liquid and solid phases much closer to the real status and predict the segregation more accurately for alloy solidification, the finite diffusion of solute element in the solid phase was considered. The modified models, such as the Brody-Flemings model [17], Clyne-Kurz model [18], Ohnaka model [19], Voller-Beckermann (V-B) model [20] and Ueshima et al.’s model [21], were developed. In these microsegregation models, the solute partition coefficient (ki), which determining the redistribution trend and segregation attribute of solute element during an alloy solidification, is an important physical parameter, and it has a significant effect on the prediction results [22,23]. Therefore, the accuracy of the ki is very important. In fact, the ki is varied with the composition [24], temperature [25], inclusion precipitation [26] and phase composition [27] during alloy solidification. So, applying the changed ki in the prediction of segregation is important and necessary. However, in previous segregation studies [14,15,28], the ki in the microsegregation model were usually applied as a constant, which unfitted with the reality. Usually, the ki could be obtained by experimental measurement [25,29] and theoretical calculation [30,31]. Although the ki gained by experiment was intuitionistic and reliable, it still has some limitations because of the complex experimental conditions and heavy workload [32]. The theoretical calculation for ki was based on the thermodynamic equilibrium of alloy solidification. This method was more simple and efficient, and its accuracy has been verified by previous studies [24,30].
Besides, during the solidification process of steel, the solute segregation at the solidification front would promote the increasing in solute concentration, which might lead to the precipitation of inclusions [33]. In response, the precipitation of inclusions would consume the solute concentration and eventually influence the solute segregation [28,34,35]. That is, there is a strong interaction between the solute segregation and inclusion precipitation, especially for the high-sulfur steel which would precipitate with a large number of MnS inclusions during solidification [3]. So, to predict the solute segregation more accurately, the influence of inclusion precipitation should not be neglected. Moreover, the solidification path and phase transition of steel, which were determined by the composition of molten steel, have a great effect on the solidification temperature range and phase composition. Thus, the microsegregation would be also affected by the solidification path and phase transition [15,23], because the factors of ki and inclusion precipitation, which could influence the segregation, were both affected by the temperature and phase composition during steel solidification [27,36]. Therefore, studying on the influencing relationship and mechanism between the phase transition and microsegregation will be helpful to explore ways to improve segregation for steel solidification.
Although the effects of solute partition coefficient [22,23], inclusion precipitation [28,34], and solidification path [15,23] on solute segregation were explored in previous studies, the factors considered were isolated and incomprehensive. In particular, there is a lack of in-depth study on the quantitative effects of solidification path and phase transition on the solute partition coefficient, inclusion precipitation, and microsegregation. In current study, based on V-B model, a microsegregation model coupled with inclusion precipitation was developed to investigate the solute microsegregation for high-sulfur steel solidification. Furthermore, the temperature and phase dependence of ki, which was calculated by the thermodynamic model of ki, was applied in the coupling microsegregation model. By the coupling model, the influences of the ki and inclusion precipitation on the microsegregation were explored and discussed. What is more, the effects of solidification path and phase transition of the high-sulfur steel were quantified for the phase composition, solute partition coefficients, inclusion precipitation and microsegregation.
Currently, various fundamental microsegregation models were developed to explore the solute segregation for steel solidification [[15], [16], [17], [18], [19], [20], [21],37]. Among them, the V-B model was suitable for a broad range of cooling conditions and considered the influence of dendrite coarsening on solute diffusion, and it was widely recognized and applied [15,23]. The V-B model is described as follows:
$ C_{L,i}^{f_s}$= $C_{L}^{0} (1+f_{s}(\beta_{i}k_{i}-1))^{(1-k_{i})/(\beta_{i}k_{i}-1)}$ (1)
where $ C_{L,i}^{f_s}$ is the solute concentration in the liquid phase, $ C_{L,i}^{0}$ is the initial solute concentration in the molten steel, fs is the solid fraction, ki is the solute partition coefficient. βi is the back-diffusion parameter, and it is defined as [15,18]:
βi=2α$^{+}_{-}$i+(1-exp(-$\frac{1}{\alpha _{i}^{+}}$)-exp(-$\frac{1}{2 \alpha _{i}^{+}}$) (2)
An additional term was added on to the Fourier number to consider the influence of dendrite coarsening on microsegregation by V-B model [20]:
$\alpha _{i}^{+}$ =2(αi+αC),αC=0.1 (3)
where αi is the Fourier number, and αC is the additional term of the αi.
αi=$\frac{D_{i}^{S}·t_{f}}{X^{2}}$ (4)
where $D_{i}^{S}$ is the solute diffusion coefficient in the solid phase, tf is the local solidification time, and X is equal to the half of secondary dendrite arm spacing.
tf=$\frac{T_{L}- T_{S}}{R_{C}}$ (5)
X=λ2/2 (6)
where TS is the solidus temperature while TL is the liquidus temperatures, K; RC is the cooling rate of the steel, K/s; λ2 represents the secondary dendrite arm spacing, μm, and it is estimated by an empirical relationship as follow [15]:

Based on the V-B model, the solute concentration profile could be predicted during steel solidification. But, in previous studies [15,21,28], the solute partition coefficient (ki) in Eq. (1) was usually adopted as a constant, which was not in accordance with the facts that the ki was varied with the temperature and solidified phases during solidification [24,25,27]. So, in current study, the changed ki, which was calculated by a solute partition coefficient thermodynamic model, was applied in Eq. (1) for the prediction of microsegregation. That is, the changed ki was adopted to predict the $D_{L,i}^{f_{s}}$ at different stages of solidification (fs, from 0 to 1). In addition, the temperature and phase dependence of solute diffusion coefficients ($D_{i}^{S}$ in Eq. (7)) were also applied in the microsegregation model. The $D_{i}^{S}$ for solutes C, Si, Mn, P, and S that in the δ and γ phases are listed in Table 1. Besides the effect of ki and $D_{i}^{S}$, the effect of inclusion precipitation on the microsegregation was also taken into account by coupling calculation.
Table 1 Solute diffusion coefficients in the δ and γ phases of steel [
| Elements | $D_{i}^{\delta}$ (cm2/s) | $D_{i}^{\gamma}$ (cm2/s) |
|---|---|---|
| C | 0.0127exp(-19450/(RT)) | 0.0761exp(-32160/(RT)) |
| Si | 8.0exp(-59500/(RT)) | 0.3exp(-60100/(RT)) |
| Mn | 0.76exp(-53640/(RT)) | 0.055exp (-59600/(RT)) |
| P | 2.9exp(-55000/(RT)) | 0.01exp (-43700/(RT)) |
| S | 4.56exp(-51300/(RT)) | 2.4exp(-53400/(RT)) |
The solute segregation and inclusion precipitation influenced each other in real time during the solidification process of steel [28]. In present study, taking the precipitation of MnS inclusion in the high-sulfur steel as example, the influencing relationship between the microsegregation and inclusion precipitation were analyzed and discussed. The precipitation of MnS inclusion in the liquid steel are based on the thermodynamic equilibrium, which are as follows [34,38].
[Mn]+[S]=MnS (8)
logKeq=4.745-$\frac{8626.7}{ T }$ (9)
Keq=$C_{L,Mn,eq}^{ f_{s}}$·$C_{L,S,eq}^{ f_{s}}$ (10)
where $C_{L,S,eq}^{f_s}$ and $C_{L, Mn,eq}^{f_s}$ are the equilibrium concentrations of S and Mn for the precipitation of MnS inclusion, respectively, T is the temperature, and R represents the ideal gas constant.
Based on Eqs. (9) and (10), the equilibrium concentration product of MnS precipitation ($C_{L,S,eq}^{f_s}$··$C_{L, Mn,eq}^{f_s}$) could be determined. At each step of solidification, if the real concentration product of S and Mn ($C_{L,S}^{f_s}$,·$C_{L,Mn}^{f_s}$), which were calculated by Eq. (1) in the microsegregation model, was exceeded the ‘$C_{L,S, eq}^{f_s}$·$C_{L, Mn,eq}^{f_s}$’, the MnS inclusion would precipitate in the steel [28]. The MnS precipitates was assumed thermodynamically stable, that is, the contents of Mn and S in the MnS inclusion were supposed to be the ideal chemical ratio [38]:
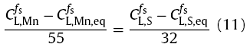
where $C_{L, S}^{f_s}$ and $C_{L, Mn}^{f_s}$ are the S and Mn concentrations in the residual liquid of steel before the precipitation of MnS inclusion, respectively. Based on the mass conservation, the precipitation amount of MnS inclusion could be calculated as follows:
$C_{L, MnS }^{f_s}$=($C_{L, S}^{f_s}$-$C_{L, S, eq}^{f_s}$)+($C_{L, Mn}^{f_s}$-$C_{L, Mn, eq}^{f_s}$) (12)
After the precipitation of MnS inclusion, the real concentrations of S and Mn in the residual liquid ($C_{L, S }^{{f_s}^{’}}$ and $C_{L, Mn }^{{f_s}^{’}}$) were equal to the equilibrium concentrations of S and Mn ($C_{L, S, eq }^{{f_s}^{’}}$ and $C_{L, Mn , eq}^{{f_s}^{’}}$), respectively. It should be noted that if the thermodynamic condition for MnS precipitation was not satisfied, there would be no MnS precipitates, and the concentrations of S and Mn would not be affected by MnS precipitation. That is, $C_{L, S }^{{f_s}^{’}}$=$C_{L, S }^{{f_s}}$ and $C_{L, Mn }^{{f_s}^{’}}$=$C_{L, Mn }^{{f_s}}$.
At the solidification step of fs, $C_{L, S }^{{f_s}^{’}}$ and $C_{L, Mn }^{{f_s}^{’}}$ would be determined by the coupling calculation of the above microsegregation and inclusion precipitation models. Then, $C_{L, S }^{{f_s}^{’}}$ and $C_{L, Mn }^{{f_s}^{’}}$ would be treated as the initial conditions to predict the solute concentration for the next step of solidification (fs + Δfs) by Eq. (1). That is, the solute concentrations for the solidification step of ‘fs + Δfs’, which before the precipitation of MnS inclusion, could be predicted as follows [34]:
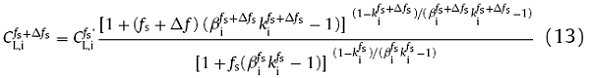
Then, the $C_{L,i}^{f_s +\Delta f_s}$ would be applied in Eqs. (9)-(12) to calculate the precipitation of MnS inclusion. The process will repeat until the end of solidification. In this way, the calculation of microsegregation and inclusion precipitation are coupled in real-time. What’s more, the ki and βi in Eq. (13) are changed at each solidification step because the tempereture and phase dependence of ki andDiS are applied in the coupling model.
If the local thermodynamic equilibrium is existed at some temperature during the steel solidification, the solute partition coefficient (ki) can be determined by the solute concentrations in the solid and liquid phases [25]. The ki is defined as:
ki=$c_{i}^{S}/ c_{i}^{L}$ (14)
where $c_{i}^{S}$ and $c_{i}^{L}$ are the solute concentrations in the solid and liquid phases of the steel, respectively. The ki essentially decides the redistribution trend of the solute element from the solid phase to the liquid phase (ki <1) or from the liquid phase to the solid phase (ki >1) during the steel solidification [25].
Based on local thermodynamic equilibrium, the chemical potential for solute element in the solid phase is equal to that in the liquid phase [27,31]:
$\mu_{i ,0}^{L}+RTlna_{i}^{L} =\mu_{i, 0}^{S}+RTlna_{i}^{S}$ (15)
where μi,0L and μi,0S are the standard chemical potentials of solute i in the liquid phase and solid phase, respectively, J/mol. aiL and aiS are the solute activities in the liquid phase and solid phase, respectively.
The solute activity (ai) is determined by the concentration and activity coefficient of solute in the liquid or solid phase:
ai=ciγi(16)
where ci is the solute concentration while γi is the activity coefficient in the liquid or solid phase.
lnγi=$ln\gamma_{i}^{0}+ε_{i}^{i}c_{i}+ε_{i}^{j}c_{j}+...+ε_{i}^{n}c_{n}$ (17)
where $\gamma_{i}^{0}$ is the solute activity coefficient in an infinite dilution solution system, $ε_{i}^{i}$, $ε_{i}^{j}$,…, and $ε_{i}^{n}$ are the interaction coefficients of solutes i, j,…, and n on solute i. Based on Eqs. (14), (15), (16), (17), the ki could be calculated as follows:
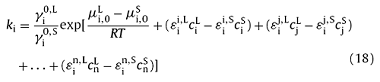
The Eqs. (14), (15), (16), (17), (18) are the theoretical basis for the calcultation of ki. In present study, the solution of ki was carried out by the thermodynamic software of FactSage6.3 which integrates the ‘Equilib’ module and contains the basic thermodynamic parameters for Fe-C-based alloy solidification [39]. Based on the initial composition of a specific steel, the liquidus and solidus temperature, the type of solidified phase (δ, γ or δ + γ), and the concentration and mass of solute element in both the liquid and solid phases, which are at a certain temperature during solidification, can be determined by the module of ‘Equilib’ in FactSage6.3. Then, the mass fraction of each phase (δ, γ or Liquid), and the ki can be calculated as follow:
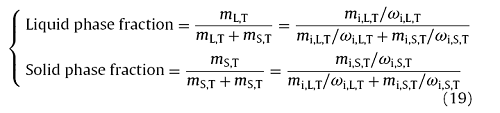
ki,T=ωi,S,T/ωi,L,T (20)
where mi,L,T and mi,S,T are the mass while ωi,L,T and ωi,S,T are the mass percentage of solute i in the liquid and solid phases, respectively, and all of them can be estimated by FactSage6.3. mL,T and mS,T are the mass of the liquid and solid phases at T during solidification, respectively. More details for the calculation of ki were descripted in literatures [23,27,31,39].
During the steel solidification, the ki was changed with the decreasing in temperature. Besides, the solidified phase might transfer from the δ phase to γ phase at a certain temperature, leading to the obvious changing on the ki, because the physicochemical properties of solute element in the δ phase were usually different from that in the γ phase. Finally, the quantified dependencies of ki in terms of temperature and phase for a specific steel solidification was obtained, and then it was applied in Eq. (13) to predict the microsegregation.
In present study, the prediction of solute microsegregation was coupled with the precipitation of inclusion. Besides, the temperature and phase dependencies of ki (and $D_{i}^{S}$) were applied. The solidification process of the steel was discreted into 10,000 qual periods (Δfs = 0.0001), and the solution of the coupling model was carried out by self-programming with Visual Basic. Fig. 1 shows the flow chart of the calculating procedure for the current coupling model.
Fig. 1. Flow chart of the current coupling model of microsegregation, inclusion precipitation, and changed solute partition coefficient and diffusion coefficient.
Firstly, based on the initial composition and cooling rate of the molten steel, the quantified dependencies of ki in terms of temperature and phase, and the solidus and liquidus temperature (TS and TL) were calculated by the FactSage6.3. Certainly, the tf and λ2 were determined by Eqs. (5) and (7). Then, the solute concentrations for one solidification step (fs = Δfs) would be predicted by the microsegregation model (Eq. (1)). Subsequently, the solute concentrations would be applied in the inclusion precipitation model to calculate the precipitation of MnS inclusion. Thus, the concentrations of S and Mn that after the precipitation of MnS inclusion ($S_{L,i}^{f_{s}^{’}}$) and the precipitation amount of MnS inclusion would be obtained. After that, a new value of the ki (and $D_{i}^{S}$) as well as the $S_{L,i}^{f_{s}^{’}}$ would be took as the initial conditions to predict the solute concentrations by Eq. (13) for the next solidification step (fs = fs + Δfs). The calculation was repeated by this way until the solidification of high-sulfur steel was over. Finally, the changing trends of the solute concentrations and the precipitation amount of MnS inclusion were obtained.
To verify the solute partition coefficient model, the partition coefficients of solutes C, Si, and Mn for various Fe-C-based alloys solidification were estimated, and the calculated results were compared with the experimental results [31], as shown in Fig. 2. The comparison results showed that the calculated ki by present model were in good agreement with the experimentals, which proved the accuracy of the solute partition coefficient model.
Fig. 2. Verification of the solute partition coefficient thermodynamic model.
Besides, Fig. 2 presents that the ki was varied with the temperature and solute composition during an alloy solidification, as previous works mentioned [27,31,40]. In other word, the ki was a variable but not a constant [27]. In present study, the temperature and phase dependencies ki was calculated firstly by the ki model, and then it was applied in the coupling model of microsegregation and inclusion precipitation. To verify the practicability of the present coupling model, the solute concentration profiles for the S1, S2, and S3 steels (in Table 2) solidification were predicted, as shown in Fig. 3. For various steels that under different cooling rates of solidification, the predicted concentration profiles of solutes C, Mn, and S by present coupling model were agreed well with the experimental and simulation results by previous works [15,28,41], which illustrated that the present coupling model was feasible and effective.
Table 2 Chemical compositions of the steels (wt%).
| Steels | C | Si | Mn | P | S | Refs. |
|---|---|---|---|---|---|---|
| S1 | 1.00 | 0.27 | 0.36 | 0.019 | 0.018 | [28] |
| S2 | 0.13 | 0.35 | 1.52 | 0.016 | 0.002 | [15] |
| S3 | 0.21 | 0.04 | 1.50 | 0.0036 | 0.0021 | [41] |
Fig. 3. Verification of the current coupling model.
The solidification path and phase transition of an alloy were determined and influenced by the alloy composition. Fig. 4 shows the phase diagram of the high-sulfur steel solidification, and it was calculated by the thermodynamic software of FactSage6.3 which integrates the module of ‘Phase Diagram’ and contains the database of ‘FSsteel’. The composition of the high-sulfur steel was 0-0.6% C, 0.03% Si, 1.05% Mn, 0.055% P, 0.28% S, and Fe (in wt%). It could be found that the Carbon (C) content has a significant effect on the phase transition temperature, phase composition (coexisting phase), and temperature range of each coexisting phase during high-sulfur steel solidification. With the increasing C content, the beginning and ending temperatures of solidification (TL and TS) both decreased gradually, and the temperature interval between the beginning and ending of solidification (TL-TS) increased. According to the difference in phase transition during solidification, the solidification path of high-sulfur steel could be divided into four kinds. The changing of the phase composition for each solidification path were classified as follow:
Path I, 0-0.07 wt% C: L → L + δ → δ
Path II, 0.07-0.14 wt% C: L → L + δ → L + δ + γ → δ + γ
Path III, 0.14-0.48 wt% C: L → L + δ → L + δ + γ → L + γ → γ
Path IV, ≥ 0.48 wt% C: L → L + γ → γ
Fig. 4. The phase diagram of high-sulfur steel solidification.
Under the ‘Path I’ (corresponding to C ≤ 0.07 wt%), there was only L + δ coexisting phase in the mushy zone (solid-liquid coexisting zone) during solidification. Under the ‘Path II’ (0.07-0.14 wt% C), there were L + δ and L + δ + γ coexisting phases in turn during solidification, and with the increasing C content, the temperature interval of the L + δ coexisting phase decreased while the temperature interval of the L + δ + γ coexisting phase increased. Under the ‘Path III’ (0.14-0.48 wt% C), there were L + δ, L + δ + γ, and L + γ coexisting phases in turn during solidification, and with the increasing C content, the temperature intervals of the L + δ and L + δ + γ coexisting phases both decreased while the temperature interval of the L + γ coexisting phase increased. But, under the ‘Path IV’ (C > 0.48 wt%), there was only L + γ coexisting phase during solidification. In addition, the MnS inclusion could precipitate in the mushy zone under all the four solidification paths, because of the high S content in the high-sulfur steel. Based on Fig. 4, the details of the phase transition temperature, phase composition (coexisting phases), and temperature range of each coexisting phases for high-sulfur steel solidification that under different C contents (corresponding to solidification paths I, II, III, and IV) are shown in Table 3.
Table 3 Phase transition temperature, coexisting phase, and temperature range of each coexisting phase for high-sulfur steel under different solidification paths.
| Path | C (wt%) | L+δ range (K) | ΔTL+δ | L+δ + γ range (K) | ΔTL+δ+γ | L+γ range (K) | ΔTL+γ | TL-TS | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| $T_{start}^{\delta+L}$ | $T_{end}^{\delta+L}$ | $T_{start}^{\delta+\gamma+L}$ | $T_{end}^{\delta+\gamma+L}$ | $T_{start}^{\gamma+L}$ | $T_{end}^{\gamma+L}$ | ||||||
| I | 0.02 | 1793 | 1760 | 33 | - | - | - | - | - | - | 33 |
| 0.04 | 1791 | 1751 | 40 | - | - | - | - | - | - | 40 | |
| 0.07 | 1789 | 1739 | 50 | - | - | - | - | - | - | 50 | |
| II | 0.11 | 1785 | 1742 | 43 | 1742 | 1735 | 7 | - | - | - | 50 |
| 0.135 | 1783 | 1743 | 40 | 1743 | 1732 | 11 | - | - | - | 51 | |
| III | 0.16 | 1781 | 1743 | 38 | 1743 | 1737 | 6 | 1737 | 1728 | 9 | 53 |
| 0.25 | 1774 | 1747 | 27 | 1747 | 1744 | 3 | 1744 | 1712 | 32 | 62 | |
| 0.35 | 1765 | 1751 | 14 | 1751 | 1750 | 1 | 1750 | 1694 | 56 | 71 | |
| IV | 0.5 | - | - | - | - | - | - | 1752 | 1668 | 84 | 84 |
Fig. 5 presents the evolution of the mass fraction of liquid (L), δ, and γ phases (calculated by Eq. (19)) during high-sulfur steel solidification. For ‘Path I’ (0.06 wt% C), with the decreasing temperature, the δ phase formed gradually in the liquid phase, and the δ phase fraction gradually increased from 0 to 1. That is, only L → δ phase transition occurred during solidification. For ‘Path II’ (0.135 wt% C), there was only δ phase generated in the liquid phase at the initial stage of solidification (T > 1744 K), and the δ phase fraction increased gradually with the decreasing temperature. The maximum of the δ phase fraction was around 0.8. Then (T < 1744 K), the peritectic reaction (L + δ → γ phase) occurred, leading to the rapidly decreasing in δ phase fraction while the γ phase fraction increased rapidly. The solidified phase was dominated by the γ phase when the solidification was over. For ‘Path III’, at the initial stage of solidification, the phase transition process was similar to ‘Path II’, but the temperature range of the peritectic reaction was narrower. Besides, the δ phase disappeared when the peritectic reaction was end. Then, the L → γ phase transition occurred, and the γ phase fraction increased rapidly with the decreasing temperature. In addition, under the ‘Path III’, with the increasing C content (0.20, 0.25, and 0.35 wt% C), the temperature interval that existence of δ phase reduced, and the maximum of δ phase fraction decreased. The phase transition of ‘Path III’ was mainly dominated by L → γ phase transition for a higher C content. For ‘Path IV’ (0.55 wt% C), there was only L → γ phase transition, and the γ phase fraction gradually increased from 0 to 1, while the δ phase fraction was always equal to zero during solidification. The above analysis illustrated that the phase transition as well as the liquid, δ and γ phase fractions that under different solidification paths of high-sulfur steel were quite different.
Fig. 5. The evolution of the mass fractions of liquid, δ and γ phases for high-sulfur steel under different solidification paths. (a) liquid phase, (b) δ phase, and (c) γ phase.
As the temperature decreased in the mushy zone during solidification, δ phase would generate and maintain at high temperature region. Then, peritectic reaction occoured and the δ phase transferred into γ phase. The proportion of phase maintained temperature region for δ and γ phases (Pδ and Pγ) in high-sulfur steel under different solidification paths are shown in Fig. 6. Under the ‘Path I’, there was only L → δ phase transition, leading to only δ phase was generated during solidification. So, the Pδ was 100% while the Pγ was 0. Contrary to ‘Path I’, there was only L → γ phase transition during solidification under the ‘Path IV’, and the Pγ was 100% while the Pδ was 0. Besides, the C content has no effect on the Pδ and Pγ that under the ‘Path I’ and ‘Path IV’, respectively.
Fig. 6. The proportion of phase maintained temperature region for δ and γ phases (Pδ and Pγ) in high-sulfur steel under different solidification paths. (a) Sketch map of Pδ and Pγ, and (b) Pδ and Pγ with C content.
Nevertheless, under the ‘Path II’ and ‘Path III’, both the δ and γ phases generated in the mushy zone during solidification, and the phase transition and phase composition were easily affected by the C content. As a result, the Pδ and Pγ were both changed with the C content, which were monotonic relations. The fitting formulas between the Pδ (and Pγ) and the C content were as follow:
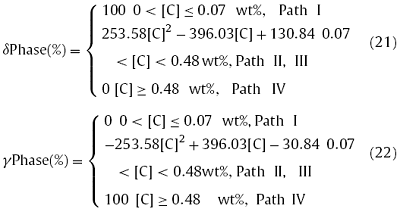
The solute partition coefficient (ki) was not a constant, but varied with the composition, temperature, and phase composition during the solidification process of an alloy [27,31,40]. Fig. 7 shows the variation of the ki for high-sulfur steel that under different solidification paths. Notes, $k_{i}^{\delta/L }$ represents the ki between the δ and liquid phases in the L + δ coexisting phase, $k_{i}^{\delta+\gamma/L }$ represents the ki between the δ + γ and liquid phases in the L + δ + γ coexisting phase, and $k_{i}^{\gamma/L }$ represents the ki between the γ and liquid phases in the L + γ coexisting phase during solidification. It could be found that kC was mainly influenced by the phase composition. The temperature almost has no effect on $k_{C}^{\delta /L }$, and $k_{C}^{\delta /L }$L was essentially unchanged under different solidification paths. But with the decreasing temperature, $k_{C}^{\delta+\gamma /L }$ increased rapidly while $k_{C}^{\gamma /L }$ increased slowly. Besides, as a whole, the $k_{C}^{\gamma /L }$ was greater than the $k_{C}^{\delta /L }$, as shown in Fig. 7(a). Unlike kC, ksi was mainly affected by the temperature, while the effect of phase composition was small. The kSi increased with the decreasing temperature under all of the four solidification paths. Fig. 7(c) illustrates that kP was mainly affected by the phase composition. The temperature has little effect on both the $k_{P}^{\delta/L} $ and $k_{P}^{\gamma/L}$, and both the kPδ/L and $k_{P}^{\gamma/L}$ were essentially unchanged under different solidification paths. As a whole, the $k_{P}^{\delta/L} $ was greater than the $k_{P}^{\gamma/L}$. But, $k_{P}^{{\delta +\gamma/L}}$ was decreased rapidly with the decreasing temperature. The change rules of kS were similar to kP. Nevertheless, the kMn was influenced by both the temperature and phase composition during solidification, as shown in Fig. 7(d). With the decreasing temperature, both the $k_{Mn}^{\delta/L}$ and $k_{Mn}^{\gamma/L}$ decreased while the $k_{Mn}^{\delta+\gamma /L}$ increased. The solidification path has some effect on the $k_{Mn}^{\gamma /L}$ and $k_{Mn}^{\delta +\gamma /L}$ but little effect on the $k_{Mn}^{\delta /L}$.
Fig. 7. The variation of solute partition coefficients for high-sulfur steel under different solidification paths: (a) kC, (b) kSi, (c) kP and kS, and (d) kMn.
In brief, Fig. 7 shows that during the solidification process of high-sulfur steel, kC, kP, and kS were mainly influenced by phase composition and kSi was primarily by temperature, while kMn depended on both phase composition and temperature. The changing of $k_{i}^{\delta+\gamma /L}$ with temperature were more significant than that for $k_{i}^{\delta /L}$ and $k_{i}^{ \gamma /L}$. The $k_{i}^{\delta /L}$ were usually different from the $k_{i}^{\gamma /L}$. In addition, from the ‘Path I’ to ‘Path IV’, the change interval of ki were quite different because of the difference in the temperature range and phase composition for the steel solidification. In other words, the solidification path and phase transition influenced the temperature range and phase composition, eventually leading to the difference in ki.
The C content has a great effect on the solidification path of high-sulfur steel, leading to the difference in ki. Fig. 8 presents the quantitative effect of C content on the average partition coefficients of solutes C, Si, Mn, P, and S ($k_{i}^{ave} $, the average value of ki during solidification) for high-sulfur steel that under different solidification paths. Fig. 8(a) shows that under the ‘Path I’ and ‘Path IV’, that is, when the solidified phase was only δ or γ phase, the C content has almost no effect on the $k_{C}^{ave} $. The $k_{C}^{ave} $ under the ‘Path I’ was around 0.150, which was less than that under the ‘Path IV’ (0.325). Nevertheless, under the ‘Path II’ and ‘Path III’, the $k_{C}^{ave} $ linearly increased with the increasing C content (or the decreasing Pδ). Under different solidification paths, the fitting formulas between the $k_{C}^{ave} $ and the C content were as follows:
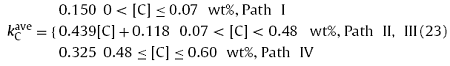
Fig. 8. The average partition coefficient of solutes (kiave) for high-sulfur steel that under different solidification paths. (a) C, (b) Si, (c) P and S, and (d) Mn.
Fig. 8(b) shows that the $k_{Si}^{ave}$ monotonously increased with the increasing C content under all the solidification paths of I, II, III, and IV. The fitting formulas were as follows:

Like $k_{C}^{ave}$, the C content has almost no effect on the $k_{P}^{ave}$ under both the ‘Path I’ and ‘Path IV’, and the kPave under the ‘Path I’ was greater than that under the ‘Path IV’. Nevertheless, the $k_{P}^{ave}$ linearly decreased with the increasing C content (or the decreasing Pδ) under both the ‘Path II’ and ‘Path III’. Besides, the change rule of the $k_{S}^{ave}$ with C content was similar to the $k_{P}^{ave}$, as shown in Fig. 8(c). The fitting formulas of the $k_{P}^{ave}$ (and $k_{S}^{ave}$) with C content were as follows:
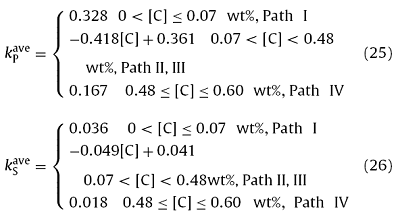
Fig. 8(d) shows that there was a complex change rule for the $k_{Mn}^{ave}$. The $k_{Mn}^{ave}$ under different solidification paths were quite different. The fitting formulas were as follows:

Fig. 8 indicates that for high-sulfur steel solidification, the C content has little effect on the $k_{C}^{ave}$, $k_{P}^{ave}$, and $k_{S}^{ave}$ that under both the ‘path I’ and ‘path IV’. But under the ‘path II’ (and ‘path III’), with the increasing C content (or the decreasing Pδ), kCave increased linearly while $k_{P}^{ave}$ and $k_{S}^{ave}$ decreased linearly. Besides, $k_{Si}^{ave}$ increased monotonously with the increasing C content under all the four solidification paths. The effect of solidification path and C content on the $k_{Mn}^{ave}$ were more complicated, and the $k_{Mn}^{ave}$ showed downtrend with the increasing C content as a whole.
Under the paths ‘I’ and ‘IV’, there were only L + δ and L + γ coexisting phases during solidification, respectively, and the C content could only affect the temperature range but without affect the phase composition. As mentioned in Fig. 7, kC, kP, and kS were mainly affected by the phase composition. In the same phase composition (L + δ or L + γ coexisting phase), the effect of temperature on all the kC, kP, and kS were small, and the kC, kP, and kS were almost unchanged. So, the C content has almost no effect on the $k_{C}^{ave}$, $k_{P}^{ave}$, and $k_{S}^{ave}$ that under the paths ‘I’ and ‘IV’, as shown in Fig. 8(a) and Fig. 8(c). But, under the paths ‘II’ and ‘III’, not only the temperature range was affected by the C content but also the phase composition, and the Pδ monotonously decreased with the increasing C content. Moreover, the $k_{C}^{\gamma/L}$L was greater than the $k_{C}^{\delta/L}$, while the $k_{P}^{\ gamma /L}$ and $k_{S}^{\ gamma /L}$ were less than the $k_{P}^{\delta/L}$ and $k_{S}^{\delta/L}$, respectively. So, with the increasing C content, the kCave linearly increased while the $k_{P}^{ave}$ and $k_{S}^{ave}$ linearly decreased. For kSi, it was mainly affected by temperature and increased with the decreasing temperature during solidification. Due to the temperature range of solidification decreased with the increasing C content, the kSiave increased with the increasing C content under all the paths ‘I, II, III, and IV’. Nevertheless, kMn was influenced by both the temperature and phase composition during high-sulfur steel solidification, leading to the effect of C content on the $k_{Mn}^{ave}$ was multifactorial and not a monotonous relation under different solidification paths.
Based on the coupling model of solute microsegregation and inclusion precipitation, and applying the temperature and phase dependencies of ki (in Fig. 7), the precipitation of MnS inclusion in the high-sulfur steel that under different solidification paths were investigated, as shown in Fig. 9. It is found that there were no MnS precipitates until fs = 0.40-0.55. Then, the precipitation amount of MnS inclusion increased gradually. In addition, with the increasing C content, that is, from solidification ‘path I’ to ‘path IV’, the precipitation starting time of MnS inclusion was advanced and the final precipitation amount of MnS inclusion increased, as shown in Fig. 10. Moreover, when the C content was between 0.07 and 0.16 wt%, the effect of C content on the final precipitation amount of MnS inclusion was more significant. The final precipitation amount of MnS inclusion under different solidification paths were mainly between 1.35 and 1.7 wt%.
Fig. 9. The precipitation of MnS inclusion in high-sulfur steel under different solidification paths.
Fig. 10. The precipitation starting time and final precipitation amount of MnS inclusion in high-sulfur steel under different solidification paths.
During the solidification process of high-sulfur steel, the precipitation of MnS inclusion was determined by both the solute concentration and temperature. The higher the solute concentrations of S and Mn, and the lower the temperature, the larger the precipitation amount of MnS inclusion. In the high-sulfur steel, the initial content of solute Mn (and solute S) that under different solidification paths were the same, leading to the precipitation of MnS inclusion was mainly determined by the temperature. From solidification ‘path I’ to ‘path IV’, the temperature range of the high-sulfur steel solidification decreased as a whole (as shown in Fig. 4 and Table 3), resulting in the increasing in the precipitation amount of MnS inclusion. Besides, when the C content was between 0.07 and 0.16 wt%, the peritectic reaction (L + δ → γ) occurred at the last stage of solidification, leading to the changing on the phase composition, which would have a great effect on the ki and microsegregation. As a result, the precipitation of MnS inclusion was significantly affected.
The phase composition and temperature affected the ki, thus affecting the solute microsegregation during the solidification process of steel. Fig. 11 displays the variation of solute concentrations in the liquid phase for high-sulfur steel solidification (path ‘III’, 0.16 wt% C). It can be found that the concentrations of solutes C, S, Si, and Mn all increased gradually across the whole stages of solidification. But, in different solidification stages, that is, in different coexisting phases, the changing trends of solute concentrations were different due to the differences in solute partition coefficients. As shown in Fig. 11(a) (and Fig. 11(c)), the increasing trends of C (and Si) concentrations in the L + δ, L + δ + γ, and L + γ coexisting phases were fluctuated because of the changing and difference in kC (and kSi). But for solutes Mn and S, the changing trends of concentrations during solidification were influenced not only by the microsegregation but also by the precipitation of MnS inclusion. The segregation promoted the increasing in concentrations while the precipitation of MnS inclusion consumed and reduced the concentrations of solutes Mn and S, leading to a sudden decreasing in Mn concentration once the MnS inclusion started to precipitate. But the S concentration was continued to increase after the precipitation of MnS inclusion, as shown in Fig. 11(b) and Fig. 11(d). This is because the kS was small and the increasing effect on the S concentration by S segregation was more stronger than the decreasing effect by MnS precipitation, and the solute S was excessive relative to the solute Mn.
Fig. 11. The predicted solute concentration profiles for high-sulfur steel solidification by current coupling model (0.16 wt% C, path III). (a) C, (b) S, (c) Si, and (d) Mn.
The temperature range and phase composition of high-sulfur steel under different solidification paths were quite different, resulting in the difference in ki and MnS precipitation, thus affecting the microsegregation. Fig. 12(a) shows that when the solid fraction was less than 0.6 (fs < 0.6), there was little difference in the P concentrations between different solidification paths, and the P concentrations increased slowly. But, when fs > 0.8, the P concentrations under the paths II, III, and IV were all higher than the P concentration under the path I. This is because there was L + γ coexisting phase under the paths II, III, and IV, while there was L + δ coexisting phase under the path I at the last stage of solidification, and $k_{P}^{\gamma/L}$ < $k_{P}^{\delta/L}$. As a result, the P segregation under the paths of II, III, and IV were stronger than that under the path I, because the smaller the ki, the higher the solute concentration. In addition, at the last stage of high-sulfur steel solidification, the kP under path III was basically the same as the kP under path IV, and the kP under paths III and IV were both less than the kP under path II (as shown in Fig. 7(c)), leading to the difference in P concentration between the paths III and IV was small, and the P concentrations under paths III and IV were both higher than the P concentration under path II. Fig. 12(a) illustrates that the effect of ki on the solute concentration was more obvious at the last stage of solidification.
Fig. 12. The variation of solute concentrations under different solidification paths of high-sulfur steel. (a) P, and (b) Mn.
During the solidification process of high-sulfur steel, the Mn concentration was affected by both the ki and MnS precipitation. Fig. 12(b) shows that in the initial stage of solidification (fs < 0.4), there was almost no difference in Mn concentrations under different solidification paths, and the Mn concentrations increased gradually. Nevertheless, the Mn concentration began to reduce once the MnS inclusion started to precipitate. It was because the consumption of Mn concentration by MnS precipitation was higher than the enrichment by segregation. Moreover, the changing trends of Mn concentrations that under different solidification paths were different once MnS inclusion began to precipitate. The higher the C content, the lower the Mn concentration. This is because the temperature range and phase composition that under different solidification paths were different, resulting in the differences in the ki, MnS precipitation, as well as the Mn concentration.
Fig. 11, Fig. 12 illustrate that the change and difference of the ki at different solidification stages have a great effect on the solute concentration and microsegregation. Such effect of the ki was more significant at the last stage of solidification. The solidification path of high-sulfur steel affected the temperature range and phase composition, thus affecting the ki and microsegregation. Besides, the precipitation of inclusion caused a significant reduction in the solute concentration.
The solute microsegregation ratio (CL/C0) under different solidification paths of high-sulfur steel are presented in Fig. 13. It is found that when the C content was less than 0.07 wt%, that is, under the path I, the C content has almost no effect on the microsegregation ratio of solute C, while the microsegregation ratio of solute P decreased slightly with the increasing C content. However, increasing the C content within the interval 0.07-0.16 wt% (corresponding to the ‘Fluctuating region’ in Fig. 13), the microsegregation ratio of solute C decreased rapidly while the microsegregation ratio of solute P increased rapidly. When the C content was more than 0.16 wt%, with the increasing C content, the microsegregation ratio of solute C decreased slightly while the microsegregation ratio of solute P increased slightly, and the overall variation of solutes C and P were both insignificant. From the paths I to IV, that is, when the C content was ranged from 0 to 0.6 wt%, the microsegregation ratio of solute Mn decreased monotonously, but the decreasing magnitudes under different solidification paths were different, especially when the C content was between 0.07 and 0.16 wt%. Similarly, with the increasing C content, the microsegregation ratio of solute S has a tendency to decrease as a whole. But, the effect of C content on the microsegregation ratio of solute S was more complex when the C content was between 0.07 and 0.16 wt%.
Fig. 13. The solute microsegregation ratio under different solidification paths of high-sulfur steel. (a) C, (b) S, (c) P, and (d) Mn.
The solutes C, Mn, P, and S in steel were all positive segregation elements, that is, the ki were all less than 1 [27]. So, the larger the ki, the lower the solute microsegregation ratio. According to Fig. 7, Fig. 8, when the C content was less than 0.07 wt% (corresponding to the path I), the overall change of the kC was slight during solidification, leading to small difference in the C microsegregation that under different C contents. But, when the C content was between 0.07 and 0.16 wt%, the peritectic reaction occurred at the last stage of solidification, leading to the differences in the δ and γ phase fractions as well as the kC between different C contents. Moreover, as presented in Fig. 11, Fig. 12, the solute concentration and microsegregation were significantly affected by ki, especially at the last stage of solidification. Therefore, the effect of C content on the C microsegregation was more significant when the C content was between 0.07 and 0.16 wt%. When the C content was between 0.16 and 0.60 wt%, the difference of kC at the last stage of solidification that under different C contents were small, leading to the difference in C microsegregation between different C contents was small, as shown in Fig. 13(a). The influence of kP on the P microsegregation under different C contents were similar to the influence of kC on the C microsegregation.
The microsegregation of solutes Mn and S were influenced and determined by the kMn, kS, and MnS precipitation. During high-sulfur steel solidification, the effect of C content on the kMn was not significant, and the Mn microsegregation was mainly influenced by the precipitation of MnS inclusion. On the other hand, the higher the C content, the lower the temperature range of solidification, and the more conducive to the precipitation of MnS inclusion. Therefore, with the increasing C content, the precipitation amount of MnS inclusion increased, and the microsegregation ratio of solute Mn decreased. But the small difference of kMn between different solidification paths still has some effect on the Mn microsegregation, resulting in the reduction of Mn segregation was more significant when the C content was 0.07 - 0.16 wt%.
When the C content was between 0.07 and 0.16 wt%, the peritectic reaction occurred at the last stage of solidification, leading to the phase composition as well as the kS that under different C contents were quite different. So, the effect of kS on the S microsegregation could not be ignored. That is, the S microsegregation was affected by both the kS and MnS precipitation, which was different from the Mn segregation. As a result, the change rule of S microsegregation with C content was more complex. But, when the C content was 0-0.07 wt% (or 0.16-0.60 wt%), the difference of kS at the last stage of solidification that under different C contents were samll, leading to the effect of kS on the S microsegregation was small. In other word, the S microsegregation was mainly affected by the MnS precipitation. So, with the increasing C content (C, 0-0.07 wt% or 0.16-0.60 wt%), the microsegregation ratio of solute S decreased because the precipitation amount of MnS inclusion increased and the corresponding consumption of solute S increased.
Briefly, the above analysis and discussion indicated that during the solidification process of high-sulfur steel, the change and difference of the ki, which was caused by the difference in phase composition and temperature between different solidification paths, has a significant effect on the solute microsegregation, especially at the last stage of solidification. When the C content was between 0.07 and 0.16 wt%, that is, when the Pδ was between 75% and 100%, the peritectic reaction occurred at the last stage of solidification, leading to a significant difference in the phase composition and ki between different C contents. As a result, the microsegregation of solutes C, P, and S changed significantly with the C content. Therefore, more attention should be paid to the 0.07-0.16 wt% C for microsegregation studying of high-sulfur steel. Besides, the microsegregation of solutes Mn and S were comprehensive influenced by the kMn, kS, and MnS precipitation. The studies would help understand and improve the segregation of high-sulfur steel by controlling the solidification and precipitation in practice.
The quantitative effects of phase transition on the solute partition coefficient (ki), inclusion precipitation, and microsegregation during high-sulfur steel solidification were investigated by a coupled model of microsegregation and inclusion precipitation, wherein the quantified dependencies of ki in terms of temperature, phase and Carbon (C) content were applied. The conclusions are drawn as follows;
(1) There were four kinds of solidification paths for high-sulfur steel. The phase transition temperature, phase composition, and temperature range of each coexistent phase that under different solidification paths were quite different. When the C content was 0.07-0.48 wt%, the ‘proportion of the δ or γ phase maintained temperature region’ (Pδ or Pγ) during solidification was changed monotonically with the C content.
(2) During high-sulfur steel solidification, kC, kP, and kS were mainly affected by phase composition and kSi was primarily by temperature, while kMn depended on both phase composition and temperature. The solidification path and phase transition influenced the temperature range and phase composition, eventually leading to the difference in ki.
(3) When the solidified phase was only δ or γ phase (Pδ = 1 or Pγ = 1), the C content has small effect on the average partition coefficients (kiave) of solutes C, S, and P. But, when the solidified phase was composed of δ and γ phases (corresponding to 0.07-0.48 wt% C), with the increasing C content, kCave increased linearly while kPave and kSave decreased linearly.
(4) The peritectic reaction impacted on the phase composition and ki, leading to the change in microsegregation. Such effect of the peritectic reaction was more significant at the last stage of solidification. When the Pδ was between 75% and 100% (corresponding to 0.07-0.16 wt% C), the C content resulted in a greater effect on the microsegregation of solutes C, P, and S because of the peritectic reaction.
(5) The precipitation of MnS inclusion was affected by the solidification path, and it has a reducing effect on the Mn and S microsegregation. The Mn and S microsegregation were comprehensively influenced by kMn, kS and MnS precipitation as well.
The work was financially supported by the National Natural Science Foundation of China (Nos. 51504048, 51611130062, 51874059 and 51874060), the Natural Science Foundation of Chongqing (No. cstc2018jcyjAX0647) and the Fundamental Research Funds for the Central Universities of China (No. cqu2018CDHB1B05).

/
| 〈 |
|
〉 |